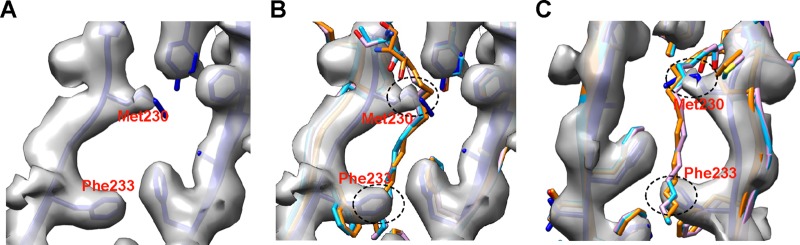
FIG 4.
Collapsed hydrophobic pocket in VP1 of BPL-inactivated CVA16. (A) In the hydrophobic pocket of the BPL-inactivated CVA16 mature virion, the side chains of Met230 and Phe233 in VP1 partially occupy the binding position for pocket factor. The positions of cryo-EM densities for the side chains of Met230 and Phe233 were determined according to the fitted CVA16 135S crystal structure (PDB 4JGY) (blue). (B) The structures in panel A were superimposed with the crystal structures of the EV71 mature virion (PDB 3VBS) (orange), the CVA16 mature virion (PDB 5C4W) (cyan), and the CVA16 empty capsid (PDB 5C9A) (pink), which all have a pocket factor, so as to illustrate where a pocket factor supposed to reside in the pocket. (C) Panel B viewed from the back. The potential clashes between pocket factor and the side chains of Met230 and Phe233 in VP1 are indicated by black dashed ovals.