Abstract
Cell proliferation, transformation, and epithelial-mesenchymal transition (EMT) are key processes involved in the development of idiopathic pulmonary fibrosis (IPF). This study investigated the regulatory factors and signaling pathways that mediate EMT in the human type II alveolar epithelial A549 cell line. A549 cells were cultured in RPMI-1640 medium and allocated to the following four groups: blank control group or treated with transforming growth factor-β1 (TGF-β1), TGF-β1 + PD 150606 (a calpain 1 inhibitor), or PD 150606. We examined E-cadherin (E-cad), α-smooth muscle actin (α-SMA), and calpain 1 mRNA transcript and protein expression levels in these four groups by performing RT-PCR and western blot analyses. The results indicated that TGF-β1 treatment significantly downregulated E-cad and upregulated α-SMA expression compared with that of the blank control group (P<0.05). TGF-β1 also enhanced calpain 1 expression compared with that of the blank control group (P<0.05). By contrast, treatment with the calpain 1 inhibitor PD 150606 increased E-cad expression and decreased α-SMA expression. Furthermore, PD 150606 treatment antagonized TGF-β1-mediated increase in Akt/phospho-Akt in A549 epithelial cells. However, TGF-β1-induced ETM was not correlated with the ERK and JNK signaling pathways. These combined results indicate that calpain 1 could regulate EMT in TGF-β1-treated A549 epithelial cells via the PI3K/Akt signaling pathway.
Keywords: Idiopathic pulmonary fibrosis, epithelial-mesenchymal transition (EMT), transforming growth factor-β1 (TGF-β1), calpain 1, PI3K/Akt signaling
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive, incurable, and fatal interstitial lung disease with unknown etiology [1,2]. The occurrence of IPF is increasing, and life expectancy is poorer than that of some cancers [3,4]. Currently, there are no pharmaceuticals or therapies that prevent IPF progression, and life expectancy is 2.5-3.5 years after diagnosis [5]. IPF also carries a burden of morbidity and poor quality of life [6]. IPF pathology causes increased deposition of extracellular matrix (ECM) and triggers the formation of fibro-proliferative foci [7]. IPF causes some cell types to transdifferentiate into activated myofibroblasts, which secrete large amounts of ECM that accumulates in lung tissues and ultimately destroys alveolar structure [8]. Therefore, cell proliferation and transformation are the key processes underlying IPF [9]. Lung epithelial cells can transdifferentiate into myofibroblasts when they acquire the mesenchymal phenotype via epithelial-mesenchymal transition (EMT) [10,11].
Many factors induce EMT, including transforming growth factor-β1 (TGF-β1), epithelial growth factor (EGF), insulin-like growth factor (IGF), and interleukin-17 (IL-17) [12]. TGF-β1 is an important factor that induces EMT under both pathological and physiological conditions by triggering EMT regulators such as hypoxia-inducible factor-1 (HIF-1), Snail, zinc finger E-box binding homeobox 1 (ZEB1), and Twist [13].
EMT is always accompanied by downregulation of epithelial cell markers, such as E-cadherin (E-cad), and upregulation of mesenchymal cell markers such as α-smooth muscle actin (α-SMA) [14,15]. Calpain 1 is a non-lysosomal cysteine protease that is selectively triggered in response to changes in calcium levels. Calpain 1 regulates cellular functions such as cell trans-differentiation, cytoskeletal remodeling, apoptosis, and cell cycle progression [16-18]. Therefore, we hypothesized that calpain 1 may participate in EMT in lung epithelial cells.
In this study, we treated A549 cells (human lung epithelial cells) with TGF-β1 and/or the calpain 1 inhibitor PD 150606 (a calpastatin analog), and analyzed changes in E-cad and α-SMA expression. We show that calpain 1 regulates EMT in TGF-β1-treated A549 epithelial cells via the PI3K/Akt signaling pathway.
Materials and methods
Cell culture
The human type II alveolar epithelial cell line, A549, was obtained from the Scientific Research Department of Wuhan University, Wuhan, China. A549 cells were cultured in RPMI-1640 medium supplemented with 10% (w/v) fetal bovine serum (FBS, HyClone, Logan, UT, USA), 100 mg/l streptomycin, and 100 U/ml penicillin in a humidified 5% CO2 atmosphere at 37°C.
Cell treatment and trial grouping
Cell cultures were adjusted to a density of 10×106 cells/ml using RMPI-1640 containing 10% (w/v) FBS, and then seeded into 6-well plates (100 μl per well). The cells were allocated into four groups: blank control group (only RMPI-1640 medium was added), TGF-β1 treatment group (5 ng/ml TGF-β1), TGF-β1 + calpain 1 inhibitor (PD 150606) treatment group (5 ng/ml TGF-β1 and 20 μg/ml PD 150606), and calpain 1 inhibitor treatment group (20 μg/ml PD 150606). TGF-β1 was purchased from Peprotech (Rocky Hill, NJ, USA). The calpain 1 inhibitor PD 150606 was purchased from Abcam (catalog no. ab141464; UK).
Total RNA isolation, cDNA synthesis, and RT-PCR analysis
Total RNA of A549 cells was extracted using the TRIzol total RNA extraction kit (catalog no. DP405; Tiangen, Beijing, China) according to the manufacturer’s instructions. The cDNA was synthesized using the miRcute miRNA cDNA first chain synthesis kit (catalog no. KR201; Tiangen) according to the manufacturer’s instructions. The mRNA of calpain-1, E-cad, and α-SMA were obtained. Gene expression was evaluated by RT-PCR (amplification conditions were as follows: 97°C for 2 min; 35 cycles of 97°C for 5 s and 55°C for 30 s) using the PrimeScript One-step RT-PCR kit ver. 2 (catalog No. RR055A; Takara Bio., Dalian, China). The primer sequences used for RT-PCR are listed in Table 1. The amplified DNA was analyzed on 1.5% agarose gels, and the images were digitally captured with a CCD camera. The results were analyzed using a gel imaging analyzer, NIH Imager beta version 2.0.
Table 1.
Primer sequences used for gene amplification
| Genes | Primer sequence | Length (bp) |
|---|---|---|
| α-SMA | Forward: TCATGGTCGGTATGGGTCAG | 258 |
| Reverse: CGTTGTAGAAGGTGTGGTGC | ||
| Calpain-1 | Forward: TGTCGGAGGAGATCATCACG | 237 |
| Reverse: TCTTGGAGGAATTGGGACCC | ||
| E-cad | Forward: CGTAGCAGTGACGAATGTGG | 175 |
| Reverse: CTGGGCAGTGTAGGATGTGA | ||
| GAPDH | Forward: AGAAGGCTGGGGCTCATTTG | 150 |
| Reverse: AGGGGCCATCCACAGTCTTC |
Western blot analysis
A549 cells were harvested and lysed in RIPA lysis buffer. The lysate was centrifuged at 12,000× g for 5 min at 4°C. The protein concentration was determined using the BCA assay kit (Pierce, Rockford, IL, USA). Equal amounts of proteins were loaded and separated by SDS-PAGE according to standard protocols and the manufacturer’s instructions (Tiangen). Subsequently, the proteins were transferred to a PVDF membrane (Millipore, Bedford, MA, USA) for 2 h at 4°C. The membranes were blocked with 5% non-fat milk in phosphate buffer saline containing Tween 20 (PBST) and washed three times with PBST (5 min per wash). Then, the membranes were incubated overnight at 4°C with one of the following antibodies in PBST: rabbit anti-E-cad monoclonal antibody (catalog no. ab40772; 1:2000, Abcam), rabbit anti-calpain 1 polyclonal antibody (catalog no. ab28257; 1:2000, Abcam), or mouse anti-α-SMA monoclonal antibody (catalog no. sc-71626; 1:4000, Santa Cruz, CA, USA). Subsequently, the membranes were washed three times with PBST (5 min per wash), and incubated for 2 h at room temperature with horseradish peroxidase-conjugated goat anti-mouse (catalog no. BA1051; 1:2000, Boster, Inc., Wuhan, China) or horseradish peroxidase-conjugated goat anti-rabbit (catalog no. BA1054; 1:2000, Boster, Inc., Wuhan, China). The membranes were examined for antibody binding using the western electrochemiluminescence (ECL) kit (catalog no. 32106; Pierce). Band intensities were detected and quantified using the AlphaEaseFC image analyzer system (Alpha Innotech, Inc., CA, USA). Relative protein expression was normalized with respect to that of GAPDH (ratio of OD values of proteins and GAPDH).
Statistical analysis
All analyses were performed using SPSS 17.0 software (IBM). Multiple comparisons were analyzed with analysis of variance (ANOVA). Pairwise comparisons between means of two groups were analyzed with Student’s t-test. Data are reported as the means ± SD (standard deviation). All experiments were performed independently and repeated at least three times. A P value less than 0.05 was considered statistically significant.
Results
TGF-β1 downregulates E-cad expression
Cells were treated with TGF-β1 to induce EMT, and mRNA transcript and protein expression levels of E-cad were examined by RT-PCR and western blotting, respectively. The results indicated that TGF-β1 treatment significantly reduced E-cad mRNA expression compared with that of the blank control group (Figure 1A, P<0.01). TGF-β1 treatment also reduced E-cad protein expression compared with that of the blank control group (Figure 1B, P<0.001).
Figure 1.
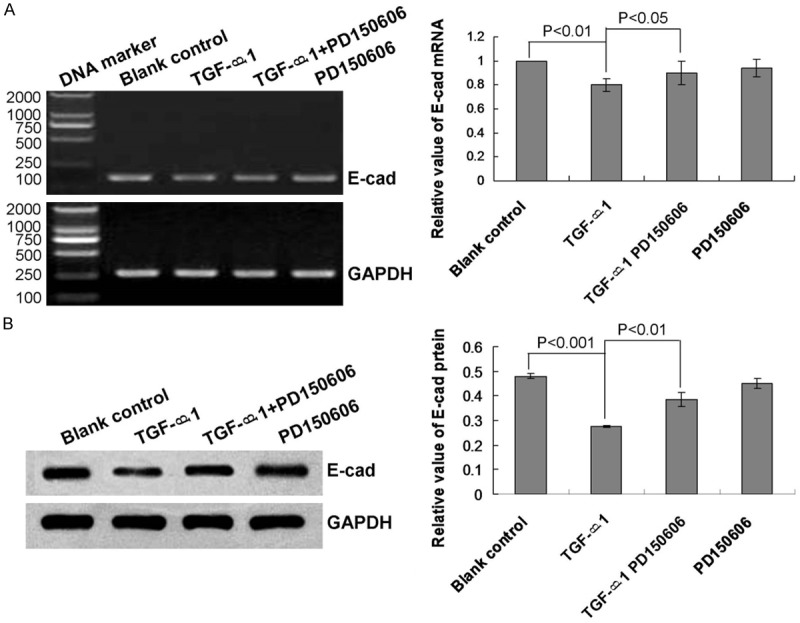
Analysis of E-cadherin mRNA transcript and protein expression levels in human lung epithelial cells. A. RT-PCR analysis of E-cad mRNA expression in the blank control, or treated with TGF-β1, TGF-β1 + PD 150606, or PD 150606. B. Western blot analysis of E-cad protein expression in the blank control, or treated with TGF-β1, TGF-β1 + PD 150606, or PD 150606. Statistical analyses and P values are given in the charts.
TGF-β1 upregulates α-SMA expression
We also tested the effect of TGF-β1-induced EMT on α-SMA transcript and protein expression levels. The results indicated that TGF-β1 treatment significantly increased α-SMA mRNA (Figure 2A, P<0.01) and protein (Figure 2B, P<0.001) expression levels compared with that of the blank control group.
Figure 2.
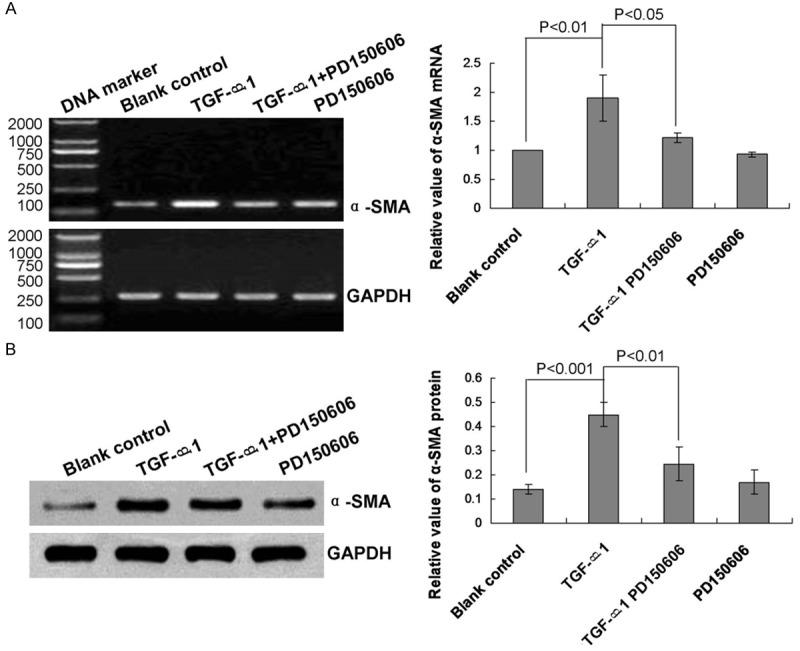
Analysis of α-smooth muscle actin mRNA transcript and protein expression levels in human lung epithelial cells. A. RT-PCR analysis of α-SMA mRNA expression in the blank control, or treated with TGF-β1, TGF-β1 + PD 150606, or PD 150606. B. Western blot analysis of α-SMA protein expression in the blank control, or treated with TGF-β1, TGF-β1 + PD 150606, or PD 150606. Statistical analyses and P values are given in the charts.
TGF-β1 enhances calpain 1 expression
We evaluated calpain 1 expression during TGF-β1-induced EMT to assess its role in lung epithelial cells. The results indicated that TGF-β1 treatment significantly enhanced calpain 1 mRNA (Figure 3A, P<0.01) and protein (Figure 3B, P<0.001) expression levels compared with that of the blank control group.
Figure 3.
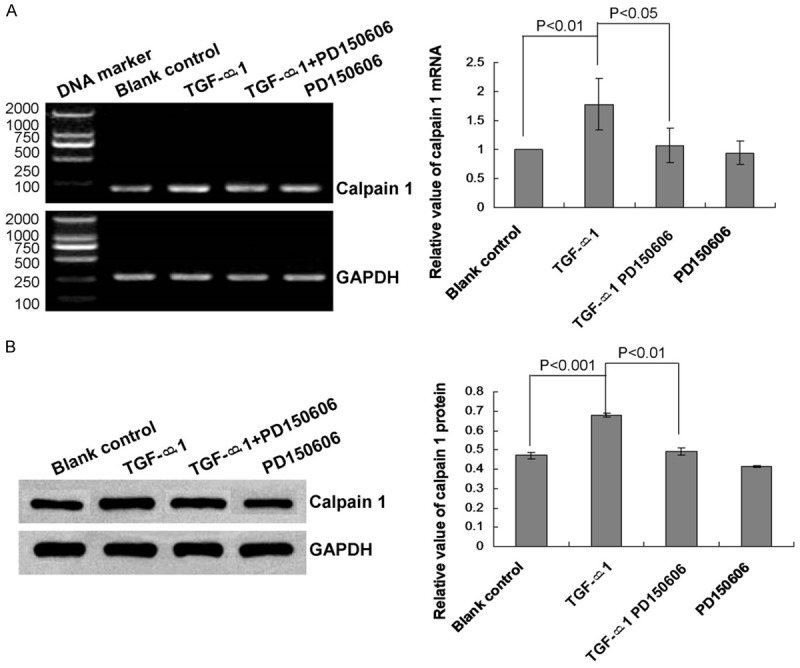
Analysis of calpain 1 mRNA transcript and protein expression levels in human lung epithelial cells. A. RT-PCR analysis of calpain 1 mRNA expression in the blank control, or treated with TGF-β1, TGF-β1 + PD 150606, or PD 150606. B. Western blot analysis of calpain 1 protein expression in the blank control, or treated with TGF-β1, TGF-β1 + PD 150606, or PD 150606. Statistical analyses and P values are given in the charts.
Calpain 1 inhibitor enhances E-cad expression and reduces α-SMA expression
We hypothesized that calpain 1 may be a specific factor associated with TGF-β1-induced downregulation of E-cad, upregulation of α-SMA, and EMT. Therefore, we treated lung epithelial cells with the calpain 1 inhibitor PD 150606 (a calpastatin analog). The results showed that PD 150606 treatment significantly attenuated the enhancement of calpain 1 mRNA and protein expression levels caused by TGF-β1 treatment (Figure 3A and 3B, P<0.05). By contrast, treatment with PD 150606 also significantly attenuated the effects of TGF-β1 on E-cad (Figure 1A and 1B, P<0.05) and α-SMA (Figure 2A and 2B, P<0.05) expression levels.
Calpain 1 inhibitor antagonizes TGF-β1-induced increase in Akt/phospho-Akt
We investigated possible mechanisms and signaling pathways involved in human lung cell EMT and TGF-β1-induced changes in calpain 1 expression levels. The results indicated that PD 150606 treatment significantly attenuated TGF-β1-induced increase in Akt/phospho-Akt expression levels (Figure 4A, 4D, and 4E, P<0.05).
Figure 4.
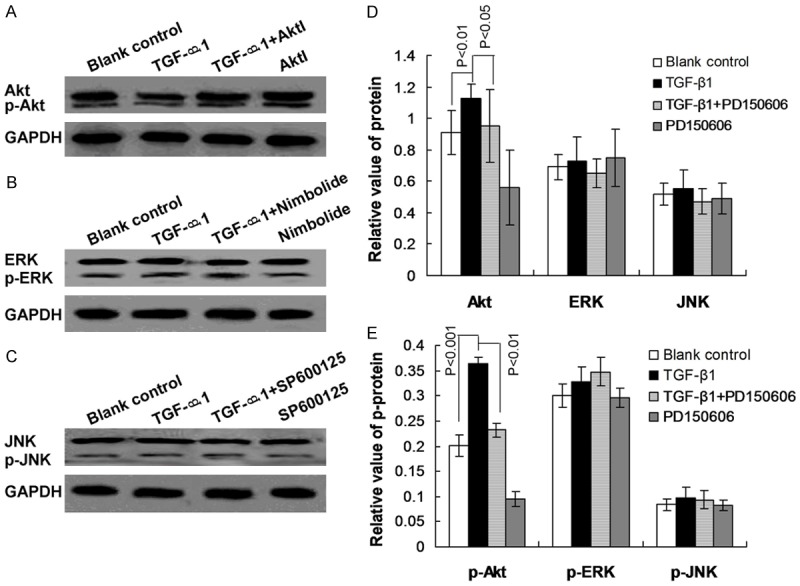
Analysis of Akt, ERK, and JNK signaling pathways during epithelial-mesenchymal transition in human lung epithelial cells. A. Western blot analysis of Akt/phospho-Akt expression in the blank control, or treated with TGF-β1, TGF-β1 + PD 150606, or PD 150606. B. Western blot analysis of ERK/phospho-ERK expression in the blank control, or treated with TGF-β1, TGF-β1 + PD 150606, or PD 150606. C. Western blot analysis of JNK/phospho-JNK expression in the blank control, or treated with TGF-β1, TGF-β1 + PD 150606, or PD 150606. D. Statistical analysis of Akt, ERK, and JNK expression levels. E. Statistical analysis of phospho-Akt, phospho-ERK, and phospho-JNK expression levels. P values are given in the charts.
We also assessed whether other signaling pathways, such as the ERK and JNK pathways, might be involved in TGF-β1-induced EMT. The results showed that the TGF-β1-induced ETM was not correlated with ERK and JNK signaling (Figure 4B-E, P>0.05).
Discussion
Epithelial-mesenchymal transition in lung cells has an important role in the transdifferentiation of lung epithelial cells to myofibroblasts [19]. EMT in lung epithelial cells is associated with decreases in E-cad levels and increases in α-SMA levels [10]. Many studies [20-22] have confirmed the critical role of EMT in the development of idiopathic pulmonary fibrosis. EMT has an important role in maintaining cellular homeostasis in the tumor microenvironment, which includes oxidative stress, inflammation, and hypoxia [10]. The human A549 alveolar epithelial cell line is a model that can be used to simulate IPF via TGF-β1-induced cell proliferation and EMT. Previous studies confirmed that TGF-β1 induces EMT [23,24]. EMT has the characteristics of an invasive and migratory cellular phenotype, which is similar to that of tumor cells with increased metastatic potential. We treated A549 cells with TGF-β1 to induce EMT and observed corresponding changes in E-cad, α-SMA, and calpain 1-expression levels.
Calpain is a ubiquitous Ca2+-regulated cysteine protease in many mammals and other organisms. Calpain also has a role in protein modification pathways that are involved in the limited proteolysis of target proteins [25]. Calpain activity is essential for cell mobility, cell cycle progression, cell migration, apoptosis, and necrosis [26,27]. Recent studies report that calpain has an important role in EMT [28,29]. Calpain is activated primarily by two different signaling pathways [30]: increased cytosolic free Ca2+ concentration and the ERK/MAPK signaling pathway activated by epithelial growth factor (EGF). Our results showed that treatment of A549 cells with TGF-β1 upregulated calpain expression, downregulated E-cad expression, and upregulated α-SMA expression. By contrast, treatment of A549 cells with the calpain inhibitor PD 150606 significantly increased E-cad expression and significantly decreased α-SMA expression. These results suggest that TGF-β1 triggers EMT by upregulating calpain 1 expression. However, the mechanism that mediates TGF-β1-induced increase in calpain 1 expression will be elucidated in future studies.
Other signaling pathways involved in EMT include the phosphoinositide 3-kinase (PI3K)/Akt pathway and the ERK and JKN MAPK pathways [31-34]. We investigated potential roles for the Akt, ERK, and JNK signaling pathways in TGF-β1-induced enhancement of calpain 1 expression during EMT in A549 cells. The results showed that PD 150606-mediated inhibition of calpain 1 expression significantly downregulated Akt/phospho-Akt expression levels. However, treatment of A549 cells with PD 150606 did not significantly affect ERK/phospho-ERK or JNK/phospho-JNK expression levels, indicating that calpain activity is not involved in ERK and JNK signaling during EMT. This result does not support a previous study, which reported that TGF-β1 regulates EMT by activating ERK and JNK signaling.
In conclusion, this study has shown that calpain 1 could regulate EMT in TGF-β1-treated A549 cells via the PI3K/Akt signaling pathway. This result provides new insights into the cellular mechanisms that mediate EMT in human lung epithelial cells and may provide therapeutic targets for clinical studies on idiopathic pulmonary fibrosis.
Acknowledgements
This study was granted by the National Natural Science Foundation of China (Grant No. 81570063).
Disclosure of conflict of interest
None.
References
- 1.Duck A, Spencer LG, Bailey S, Leonard C, Ormes J, Caress AL. Perceptions, experiences and needs of patients with idiopathic pulmonary fibrosis. J Adv Nurs. 2015;71:1055–1065. doi: 10.1111/jan.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, Cookson WO, Maher TM, Moffatt MF. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navaratnam V, Fleming KM, West J, Smith CJ, Jenkins RG, Fogarty A, Hubbard RB. The rising incidence of idiopathic pulmonary fibrosis in the UK. Thorax. 2011;66:462–467. doi: 10.1136/thx.2010.148031. [DOI] [PubMed] [Google Scholar]
- 4.Ley B, Collard HR, King TE, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 6.Schoenheit G, Becattelli I, Cohen AH. Living with idiopathic pulmonary fibrosis: an in-depth qualitative survey of European patients. Chron Respir Dis. 2011;8:225–231. doi: 10.1177/1479972311416382. [DOI] [PubMed] [Google Scholar]
- 7.Li FZ, Cai PC, Song LJ, Zhou LL, Zhang Q, Rao SS, Xia Y, Xiang F, Xin JB, Greer PA, Shi HZ, Su Y, Ma WL, Ye H. Crosstalk between calpain activation and TGF-beta 1 augments collagen-1 synthesis in pulmonary fibrosis. Biochim Biophys Acta. 2015;1852:1796–1804. doi: 10.1016/j.bbadis.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Scotton CJ, Hambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 9.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286–289. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 10.Yamada A, Aki T, Unuma K, Funakoshi T, Uemura K. Paraquat induces epithelial-mesenchymal transition like cellular response resulting in fibrogenesis and the prevention of apoptosis in human pulmonary epithelial cells. PLoS One. 2015;10:e0120192. doi: 10.1371/journal.pone.0120192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willis BC, Borok Z. TGF-beta induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 13.Argast GM, Krueger JS, Thomson S, Sujka-Kwok I, Carey K, Silva S, O’Connor M, Mercado P, Mulford IJ, Young GD. Inducible expression of TGFbeta, snail and Zeb1 recapitulates EMT in vitro and in vivo in a NSCLC model. Clin Exp Metastasis. 2011;28:593–614. doi: 10.1007/s10585-011-9394-8. [DOI] [PubMed] [Google Scholar]
- 14.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 15.Kage H, Borok Z. EMT and interstitial lung disease: a mysterious relationship. Curr Opin Pulm Med. 2012;18:517–523. doi: 10.1097/MCP.0b013e3283566721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 17.Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Glading A, Lauffenburger DA, Wells A. Cotting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Qin X, Li Y, Zhang X, Niu R, Zhang H, Cui A, An W, Wang X. MiR-133a suppresses the migration and invasion of esophageal cancer cells by targeting the EMT regulator SOX4. Am J Transl Res. 2015;7:1390–1403. [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 21.Kage H, Borok Z. EMT and interstitial lung disease: a mysterious relationship. Curr Opin Pulm Med. 2012;18:517–523. doi: 10.1097/MCP.0b013e3283566721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Zhou C, Zheng J. Cigarette smoking impairs the response of EGFR-TKIs therapy in lung adenocarcinoma patients by promoting EGFR signaling and epithelial-mesenchymal transition. Am J Transl Res. 2015;7:2026–2035. [PMC free article] [PubMed] [Google Scholar]
- 23.Ohshio Y, Teramoto K, Hashimoto M, Kitamura S, Hanaoka J, Kontani K. Inhibition of transforming growth factor beta release from tumor cells reduces their motility associated with epithelial-mesenchymal transition. Oncol Rep. 2013;30:1000–1006. doi: 10.3892/or.2013.2505. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Qi L, Liang Z, Song W, Liu Y, Wang Y, Sun B, Zhang B, Cao W. Transforming growth factor beta 1 induces EMT by the transactivation of epithelial growth factor signaling through HA/CD44 in lung and breast cancer cells. Int J Mol Med. 2015;36:113–122. doi: 10.3892/ijmm.2015.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorimachi H, Hata S, Ono Y. Impact of genetic insights into calpain biology. J Biochem. 2011;150:23–37. doi: 10.1093/jb/mvr070. [DOI] [PubMed] [Google Scholar]
- 26.Cotten SW, Kornegay JN, Bogan DJ, Wadosky KM, Patterson C, Willis MS. Genetic myostatin decrease in the golden retriever muscular dystrophy model does not significantly affect the ubiquitin proteasome system despite enhancing the severity of disease. Am J Transl Res. 2013;6:43–53. [PMC free article] [PubMed] [Google Scholar]
- 27.Van Ba H, Inho H. Significant role of calpain in proliferation/survival of bovine skeletal muscle satellite cells. In Vitro Cell Dev Biol Anim. 2013;49:785–797. doi: 10.1007/s11626-013-9666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu SM, Lin WY, Shen CC, Pan HC, Keh-Bin W, Chen YC, Jan YJ, Lai DW, Tang SC, Tien HR, Chiu CS, Tsai TC, Lai YL, Sheu ML. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPbeta and NFkB cleavage. J Pineal Res. 2016;60:142–154. doi: 10.1111/jpi.12295. [DOI] [PubMed] [Google Scholar]
- 29.Liu SH, Lee WJ, Lai DW, Wu SM, Liu CY, Tien HR, Chiu CS, Peng YC, Jan YJ, Chao TH, Pan HC, Sheu ML. Honokiol confers immunogenicity by dictating calreticulin exposure, activating ER stress and inhibiting epithelial-to-mesenchymal transition. Mol Oncol. 2015;9:834–849. doi: 10.1016/j.molonc.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glading A, Chang DA, Lauffenburger DA, Wells A. Epithelial growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- 31.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F. Role of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh YH, Wang SW, Yeh YC, Hsiao HF, Li TK. Rhapontigenin inhibits TGF-beta mediated epithelial mesenchymal transition via the PI3K/Akt/mTOR pathway and is not associated with HIF-1 alpha degradation. Oncol Rep. 2016;35:2887–2895. doi: 10.3892/or.2016.4664. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe-Takano H, Takano K, Hatano M, Tokuhisa T, Endo T. DA-Raf-mediated suppression of the Ras-ERK pathway is essential for TGF beta 1 induced epithelial-mesenchymal transition in alveolar epithelial type 2 cells. PLoS One. 2015;10:e0127888. doi: 10.1371/journal.pone.0127888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epstein Shochet G, Tartakover-Matalon S, Drucker L, Pasmanik-Chor M, Pomeranz M, Fishman A, Lishner M. Placenta-breast cancer cell interactions promote cancer cell epithelial mesenchymal transition via TGF-beta/JNK pathway. Clin Exp Metastasis. 2014;31:961–975. doi: 10.1007/s10585-014-9683-0. [DOI] [PubMed] [Google Scholar]
- 35.Cao J, Zhang X, Wang Q, Wang X, Jin J, Zhu T, Zhang D, Wang W, Li X, Li Y, Shen B, Zhang J. Cyclic AMP suppresses TGF-beta mediated adaptive Tregs differentiation through inhibiting the activation of ERK and JNK. Cell Immunol. 2013;285:42–48. doi: 10.1016/j.cellimm.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Hubchak SC, Browne JA, Schnaper HW. Epidermal growth factor inhibits transforming growth factor beta induced fibrogenic differentiation marker expression through ERK activation. Cell Signal. 2014;26:2276–2283. doi: 10.1016/j.cellsig.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


