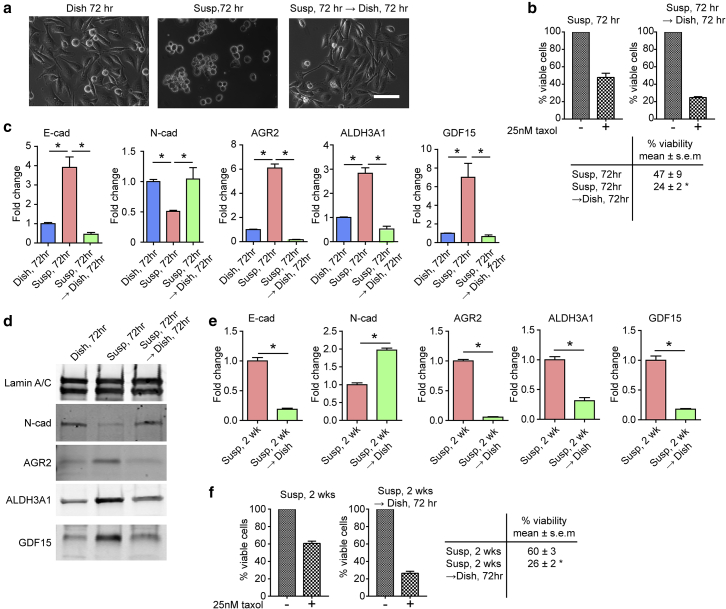
Figure 3.
Mechanosensitive phenotypic transition of PC3 cells is reversible. (a) Phase images of PC3 cells grown under different conditions as indicated for 72 h. After 72 h, PC3 cells in suspension were reseeded back onto the TCP dish and allowed to reattach for another 72 h (Susp → Dish; right). Scale bar, 100 μm. (b) Decreased sensitivity to paclitaxel in suspension culture was reversible when PC3 cells in suspension were allowed to reattach to TCP dish. Control cells were PC3 cells continuously cultured in suspension for 72 h (left). Viability was measured after an incubation with paclitaxel (25 nM) for 48 h using trypan-blue exclusion viability assay. The Susp condition for 72 h showed a viability of 49 ± 9%, while Susp 72 h → Dish 72 h had a viability of 24 ± 2%. (c) qRT-PCR analysis of E-cad, N-cad, AGR2, ALDH3A1, and GDF15 expression for PC3 cells cultured under different conditions as indicated. (d) Immunoblot assays of N-cad, AGR2, ALDH3A1, and GDF15 for PC3 cells cultured under different conditions as indicated. E-cad was not detected by immunoblot. (e) qRT-PCR analysis of E-cad, N-cad, AGR2, ALDH3A1, and GDF15. PC3 cells were cultured in suspension for 2 weeks (Susp, 2 weeks) before being allowed to reattach to TCP dish for 72 h (Susp, 2 weeks → Dish). (f) Decreased sensitivity to paclitaxel observed in Susp was sustainable in long-term suspension culture and remained reversible. PC3 cells grown in suspension for 2 weeks were allowed to reattach to TCP dish for 72 h. Control cells were PC3 cells continuously cultured in suspension for 2 weeks. Viability was measured after an incubation with paclitaxel (25 nM) for 48 h using a trypan-blue exclusion viability assay. Susp 2 weeks had a viability of 60 ± 3%, while Susp 2 weeks → Dish 72 h had a viability of 26 ± 2%. Data represents the mean ± SE with n = 3. P-values were calculated using Student’s t-test. ∗p < 0.05. To see this figure in color, go online.