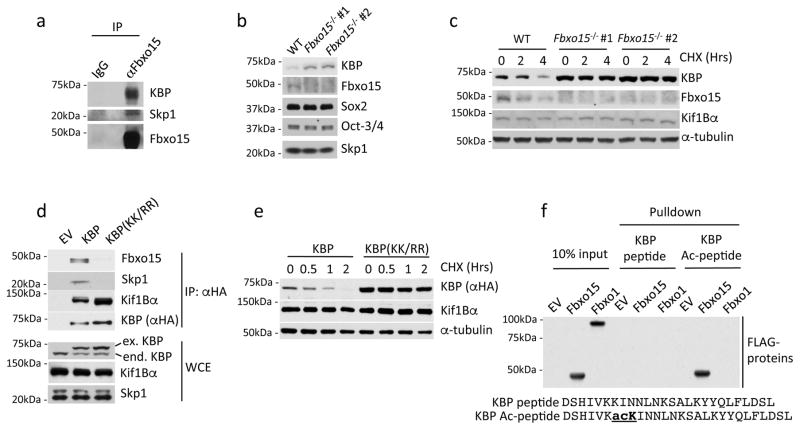
Fig. 1. KBP is an Fbxo15 substrate that is recognized in an acetylation-dependent manner.
a. Self-renewing naïve, mESCs were treated with MG132 for 4 hours prior to lysis. Lysates were immunoprecipitated with either an affinity-purified polyclonal antibody against Fbxo15 or an affinity-purified rabbit IgG, and analyzed by immunoblotting as indicated.
b. Protein extracts from wild type mESCs (WT) and Fbxo15−/− mESCs (two different clones) were immunoblotted for the indicated proteins.
c. Fbxo15−/− mESCs (two different clones) were treated with cycloheximide (CHX) for the indicated times, after which cell extracts were immunoblotted for the indicated proteins. This experiment was performed twice.
d. mESCs were infected with either an empty virus (EV) or lentiviruses expressing HA-tagged wild type mouse KBP or HA-tagged mouse KBP(KK/RR). Whole cell extracts (WCE) were immunoprecipitated (IP) with an anti-HA resin, and proteins were immunoblotted as indicated.
e. mESCs were infected with lentiviruses expressing either HA-tagged wild type mouse KBP or HA-tagged mouse KBP(KK/RR), treated with cycloheximide (CHX) for the indicated times, and total cell lysates were analyzed by immunoblotting as indicated.
f. In vitro-translated FLAG-tagged Fbxo15 and FLAG-tagged Fbxo1 were incubated with beads coupled to the indicated peptides. Beads were extensively washed, and bound proteins were immunoblotted with an anti-FLAG antibody.
Unprocessed original scans of blots are shown Supplementary Fig. 8. Unless otherwise noted, experiments were performed at least three times.