Abstract
The cellular production of free radicals or reactive oxygen species (ROS) can lead to protein, lipid or DNA modifications and tumor formation. The cellular lipids undergo structural changes through the actions of enzymes (e.g. cyclooxygenases) or free radicals to form a class of compounds called Isolevuglandins (IsoLGs). The recruitment and continued exposure of tissue to ROS and IsoLGs causes increased cell proliferation, mutagenesis, loss of normal cell function and angiogenesis. The elevated concentration of ROS in cancerous tissues suggests that these mediators play an important role in cancer development. We hypothesized that tumors with elevated ROS levels would similarly possess an increased concentration of IsoLGs when compared with normal tissue. Using D11, an ScFv recombinant antibody specific for IsoLGs, we utilized immunohistochemistry to visualize the presence of IsoLG in human tumors compared to normal adjacent tissue (NAT) to the same tumor. We found that IsoLG concentrations were elevated in human breast, colon, kidney, liver, lung, pancreatic and tongue tumor cells when compared to NAT and believe that IsoLGs can be used as a gauge indicative of lipid peroxidation in tumors.
Keywords: free radicals, isolevuglandins, lipid peroxidation, human tumors
Introduction
Free radicals are any atoms, ions or molecules that display unpaired, chemically reactive electrons. Free radicals are produced during a chronic inflammatory immune response to help fight infection but are capable of damaging DNA, RNA, proteins and lipids 1. Reactive oxygen species are free radicals that are derived from molecular oxygen and can be found as metabolic products, such as in the forms superoxide and hydrogen peroxide. Mitochondria naturally produce high levels of ROS under conditions of hypoxia, apoptosis and during elevated levels of tumor necrosis factor-α (TNF-α), a pro-inflammatory cytokine involved in mediating inflammation2. Various leukocytes and macrophages generate membrane-associated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that can endogenously catalyze the production of ROS and other metabolic products 3,4. Noninfectious causes of chronic inflammation such as those stemming from cancer chemotherapeutics, environmental toxins, industrial chemicals and ionizing radiation may exogenously contribute to the production of ROS 2,3,5,6. Under normal conditions, the production of ROS serve a protective function and are regulated via neutralizing enzymes or by small molecules that eradicate free radical intermediates. However, during abnormal or unstable conditions, the cellular systems that normally regulate or degrade ROS levels may become dysfunctional or inadequate and lead to oxidative stress and inflammation 7. Chronic inflammation and oxidative stress may be causative factors in cancer development while elevated ROS levels can lead to oxidative DNA damage, lipid peroxidation and tumor proliferation 2, 3, 5.
Oxidative stress is the imbalance caused by the production of free radicals and ROS. Proteins and lipids are especially prone to oxidative attack 3. Oxidative stress and lipid peroxidation can provoke DNA damage and genomic modifications through base changes and strand breaks that can contribute to the development of cancer 3. Prostaglandins (PGs) are a diverse class of lipid compounds derived from fatty acids and are important lipid mediators of the inflammatory immune response. Isoprostanes (IsoPs), or PG-like compounds, are formed by non-enzymatic free radical catalyzed peroxidation of arachidonic acid (AA) 8. We first reported the discovery of F2-isoprostanes (F2-IsoPs). F2-isoprostanes are derived from the oxidation of arachidonic acid and IsoP intermediates (designated H2-IsoPs). PGF2-like compounds are potent inflammatory mediators and biomarkers of lipid peroxidation and oxidative stress8. Robert Salomon previously described the rapid decomposition and rearrangement of the labile endoperoxide intermediate prostaglandin H2 (PGH2) in the cyclooxygenase (COX) pathway 9,10. H2-IsoP is also a labile endoperoxide intermediate in the IsoP pathway and is analogous to PGH2 in the prostaglandin pathway. ROS may decompose and rearrange these intermediates in the presence or absence of COX activity and can produce small (~300 mw) compounds called levuglandins (LGs) (Figure 1). As a consequence of either enzymatic (i.e. COX) or non-enzymatic free radical catalyzed peroxidation, a number of structural LG isomers can be produced that are collectively referred to as Isolevuglandins (IsoLGs)11, 12. Structurally, IsoLGs are categorized as lipid-derived levulinaldehydes13.
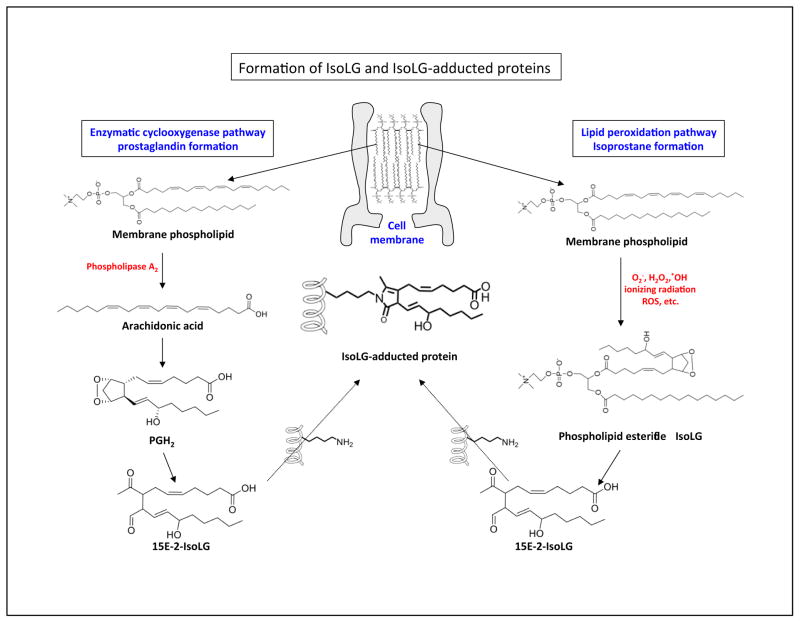
Figure 1. Formation of isolevuglandin (IsoLG) and IsoLG protein adducts.
Two pathways are used to form IsoLG protein adducts. The enzymatically derived prostaglandin pathway (left) and the free radical (lipid peroxidation) derived isoprostane pathway (right) and are depicted. (Left) Phospholipase A2 (PLA2) can cleave phospholipids to form arachidonic acid. Cyclooxygenase can oxidize arachidonic acid to form prostaglandin H2 that can subsequently undergo chemical rearrangement to form IsoLG (e.g. the 15E-2-IsoLG regioisomer). (Right) ROS stemming from mitochondria in a hypoxic tumor microenvironment can lead to lipid peroxidation. Oxidized lipids can undergo non-enzymatic chemical rearrangement to form IsoLG and IsoLG protein adducts. The chemically reactive IsoLG can then adduct to free amines (-NH2) on proteins or DNA (not depicted).
Oxidative modification of lipids can increase mutagenesis and carcinogenesis through the formation of lipid aldehydes (e.g. IsoLGs, malondialdehyde and 4-hydroxy-nonenal) that can subsequently react with DNA and other biomolecules 7, 14, 15. For example, IsoLGs are highly reactive and can covalently adduct to free amines on lysine residues to form extensive protein-protein and DNA-protein crosslinks 12, 13. The formation and interaction of lipid aldehyde byproducts with other molecules can form proinflammatory and potentially cytotoxic adducts that have been linked to an increase in malignant disease and cancer risks 7, 14, 15. These mechanisms can alter cellular pathways and gene expression as well as drive carcinogenesis by disrupting cellular homeostasis 14, 15.
In 2004, we developed a single chain fragment variable (scfv) recombinant antibody (designated D11) to 15-E2 isoketal, an IsoLG 16. The D11 scfv interacts with 15-E2 isoketal adducts on proteins independently of protein amino acid sequences. However, due to the small size and structural similarities of LGs and 15-E2 isoketal, it is probable that D11 can cross-react with all IsoLGs, regardless of their production through either a COX enzymatic or free radical pathway. The D11 scfv has been used in immunohistochemistry (IHC) to detect IsoLG-adducted proteins in cells (e.g. endothelial, epithelial, dendritic, macrophage and monocytes) stemming from inflammation or oxidative stress introduced during allergies, hyperoxia, or defects in a sodium-dependent vitamin transporter or human hypertension 17, 18, 19, 20, 21, 22. We hypothesized that elevated or abnormal levels of ROS produced in tumors leads to increased lipid peroxidation and favors IsoLG formation. We used the D11scfv in IHC to detect IsoLGs in human tumors and normal adjacent tissue (NAT) to the same tumors in biopsied samples stemming from breast, colon, kidney, liver, lung, pancreatic and tongue cancer patients. Results obtained from IHC suggest that IsoLG concentrations are prevalent and elevated in human tumor tissues in comparison to NAT from the same cancer patient.
Materials and Methods
Materials
IsoLG and IsoLG-adducted mouse serum albumin (MSA; Sigma cat# A3559) adducted at a molar ratio of 8 IsoLG:1 MSA (8:1 IsoLG/MSA) were prepared as described16. A 0.05% Tween 20 (Sigma cat# P7949) in phosphate buffered saline (PBS) was used as a wash buffer. A 5% BSA (Sigma cat# A4503) in PBS solution containing 5% normal mouse serum (Sigma cat# M5905) was used as a blocking buffer for (IHC). The D11 scfv bears an 11 amino acid sequence called the E-tag. The secondary anti-E tag monoclonal antibody coupled to peroxidase (Anti-E/HRP; G.E. Healthcare cat# 27-9413-01) and the Anti-E tag monoclonal antibody coupled to beads (Anti-E tag monoclonal antibody column G.E. Healthcare cat# 17-1362-01) were used, respectively, to detect E-tagged D11 scfv bound to IsoLG in tissue samples, and to purify E-tagged D11scfv from bacteria that were used to produce the scfv. Diaminobenzidine (DAB, Sigma cat# D3939) and hydrogen peroxide were used to visualize D11scfv:Anti-E/HRP binding in IHC assays. Hematoxylin (Sigma cat# H3136) was used as a tissue section counter-stain.
Selection, expression and purification of the anti-IsoLG D11 scfv recombinant antibody
A large (~2.9 billion member) naïve phage-displayed recombinant scfv antibody library was used to select for the scfv designated D11. Methods used to carry out D11 scfv phage selection, expression and purification have been previously described 16.
Characterization of D11 scfv binding specificity
Assays (ELISAs and IHC) used to determine D11 scfv binding specificity have been previously described, in detail 16. In summary, D11 binds to IsoLGs adducted to free amines present on proteinaceous molecules independent of amino acid sequence. D11 scfv does not bind to 15-F2t-isoprostane or to adducts stemming from other pyrrole-forming lipid peroxidation products such as 4-hydroxynon-2(E)-enal (HNE) and 4-oxononanal (ONA) 16.
Human tissue samples
Well-annotated, de-identified, formalin-fixed and paraffin-embedded human tissue samples were obtained from the Vanderbilt University Cooperative Human Tissue Network (CHTN). The CHTN operates under university-approved IRB protocols. Tumor tissue samples and normal tissue adjacent to the tumor (NAT) from the same male (M) or female (F) individual of Asian (A) Caucasian (C) or African American (B) descent were obtained for breast, colorectal (age 55 F/C, adenocarcinoma, stage IV), kidney (age 50 F/C, renal/clear cell carcinoma, stage III, grade IV), liver (age 79 F/A, hepatoma/hepatocellular carcinoma, stage II), lung (age 63 F/B, squamous cell carcinoma), pancreas (age 56 M/C, adenocarcinoma, stage IIB) and tongue (age 67 M/C, squamous cell carcinoma, stage IVA) cancer patients.
D11 scfv immunohistochemistry of human tissue samples
Six-micron thick, human tumor and NAT paraffin-embedded tissue samples were cut, and 3–4 serial sections were mounted on each glass slide. Slides were immersed in xylene twice to remove paraffin from tissue sections, then rinsed with 100% ethanol twice, re-hydrated using a gradient 95 to 35% alcohol series, then rinsed with PBS, pH 7.2. Tissue sections were incubated for 30 min at room temperature in PBS containing 30% methanol and 2% hydrogen peroxide to quench endogenous peroxidase present in tissue sections then rinsed twice with PBS. Tissue sections were blocked with PBS containing 5% BSA and 5% normal mouse serum for 45 min at room temperature to prevent non-specific antibody binding activity, then rinsed with PBS to remove excess blocking reagent. The D11 scfv was diluted to 4 μg/ml PBS and applied to tissue sections and incubated for 1 h at room temperature in a humidified container. Slides were washed 3 times with PBS (5 minutes per wash) to remove unbound scfv. The Anti-E tag/HRP monoclonal antibody was diluted 1:800 in PBS containing 0.05% Tween 20, applied to and incubated on tissue sections for 1 h at room temperature in a humidified container. Tissue sections were washed 3 times to remove unbound Anti-E/HRP. Hydrogen peroxide substrate and DAB color developer were applied to visualize D11 scfv binding to IsoLG in tissue sections. Tissue sections on slides were immersed twice in distilled water to terminate color development. Tissue sections were counter-stained with hemotoxylin for 1 min then washed in water to remove excess counter stain. Tissue sections on slides were dehydrated by passing slides through a 35–95% graded ethanol series, passed twice through 100% ethanol, then once through xylene. Cover slips were mounted on glass slides, sealed and stored at room temperature until examined by light microscopy. Images were acquired with an Olympus BH2 phase contrast microscope equipped with an Olympus Q Color 3 CCD camera and Q-Capture imaging software. All images for each section within a set of serial sections were acquired from the same location within the tissue sample using the same microscope, camera and imaging software settings.
Negative controls used for human tissue sample immunohistochemistry
The E-tagged D11 scfv and the Anti-E tag/HRP secondary antibody were used to detect IsoLGs in tumor and normal adjacent tissue (NAT) to the tumor. Three negative controls were used to ensure that D11 scfv bound specifically to IsoLG in tissue samples. Control reactions were carried out on separate tissue serial sections on the same slide, using the same conditions as used for D11 scfv and Anti-E tag/HRP tissue staining. Negative control one (Competition control): The purpose of negative control one or the competition control was to determine if D11 scfv would bind to IsoLG on tissue samples in the absence, but not in the presence, of a competing amount of purified IsoLG-adducted protein. If D11 scfv were specific for IsoLG, then D11 scfv tissue staining would be diminished in the presence of a competing amount of IsoLG-adducted protein. To carry out the competition assay, the D11 scfv (final concentration of 4 μg/ml) was mixed together with 8:1 IsoLG/MSA (final concentration of 40 μg/ml) in PBS. After a1h incubation at room temperature, the mixture was used to stain for IsoLG in tissue samples as described previously. It should be noted that D11 scfv binds IsoLG-adducted, but not non-adducted MSA in ELISA (data not presented). As such, non-adducted MSA was not used as a competitor for IHC. Negative control two: The secondary Anti-E/HRP antibody - only - was applied to tissue sections to determine if the Anti-E/HRP antibody bound non-specifically to tissue samples. Negative control three: A negative control E-tagged scfv (designated A10B: specific for rabbit IgG 23 and Anti-E tag/HRP) was used in lieu of the D11 scfv to determine if non-specific interactions occurred between E-tagged scfv in general and the tissue samples being analyzed.
Results
The D11 scfv and Anti-E tag/HRP secondary antibody were used in IHC to detect IsoLGs present in human tumor tissue and normal adjacent tissue (NAT) to the tumor. To ensure D11 and Anti-E tag/HRP binding specificity for IsoLGs, the remaining serial tissue sections mounted on the same slide were stained using one of three negative controls (see Materials and Methods). As ascertained by D11 staining, IsoLG concentration was elevated in colon, kidney and liver (Figure 2, column 1), lung, pancreas and tongue (Figure 3, column 1) tumor tissues; and markedly reduced or absent in normal adjacent tissue (NAT) (Figures 2 and 3, column 1).
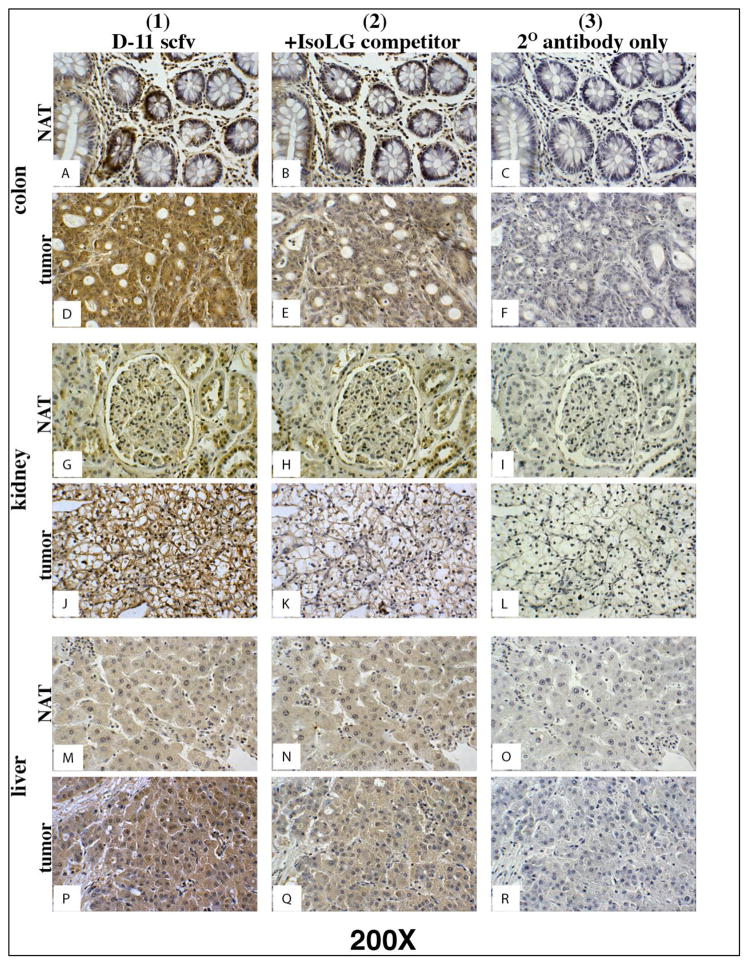
Figure 2. D11 scfv staining on serial colon, kidney and liver normal and tumor tissue.
Column 1) Tissues stained with D11 scfv; Column 2) Negative control - Tissues stained with the D11 scfv pre-incubated with a competing concentration of IsoLG-adducted albumin; Column 3) Negative control - Tissues stained only with the Anti-E tag/HRP secondary (2o) antibody. Note: Tumor cells in column 1 stain (brown) more intensely than normal (NAT) cells adjacent to the tumor. Magnification for all images depicted is 200X.
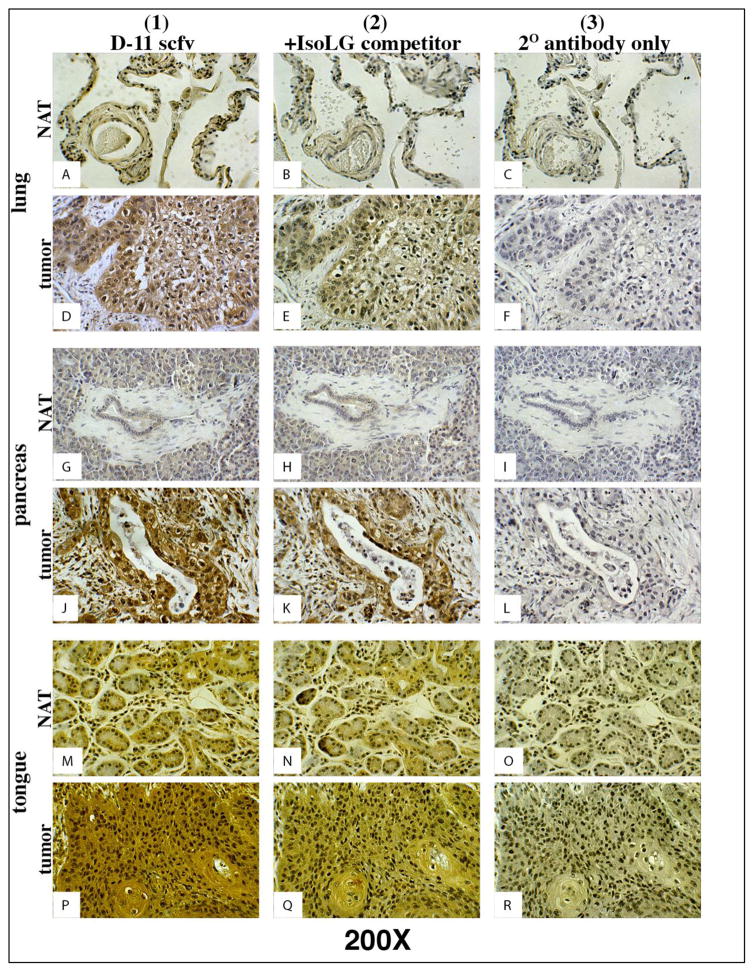
Figure 3. D11 scfv staining on serial lung, pancreas and tongue normal and tumor tissue.
Column 1) Tissues stained with D11 scfv; Column 2) Negative control - Tissues stained with the D11 scfv pre-incubated with a competing concentration of IsoLG-adducted albumin; Column 3) Negatvie control - Tissues stained only with the Anti-E tag/HRP secondary (2o) antibody. Note: Tumor cells in column 1 stain (brown) more intensely than normal (NAT) cells adjacent to the tumor. Magnification for all images depicted is 200X.
In all cases, D11 scfv binding activity was stronger when applied directly to tissue sections (Figures 2 and 3, column 1) and reduced or eliminated when pre-incubated with the IsoLG-adducted albumin competitor (Negative control one) prior to use for staining on all normal and human tissue sections (Figures 2 and 3, column 2). The Anti-E/HRP secondary antibody alone (Negative control two, Figures 2 and 3, column 3) and the irrelevant A10B scfv in the presence of the Anti-E/HRP secondary antibody (Negative control three, data not presented) did not stain any tumor or normal tissue samples. These results suggest that D11 scfv staining was specific for IsoLGs and that IsoLG concentration was elevated in human tumor tissue in comparison to normal adjacent tissue for colon, kidney, liver, lung, pancreas and tongue tumors.
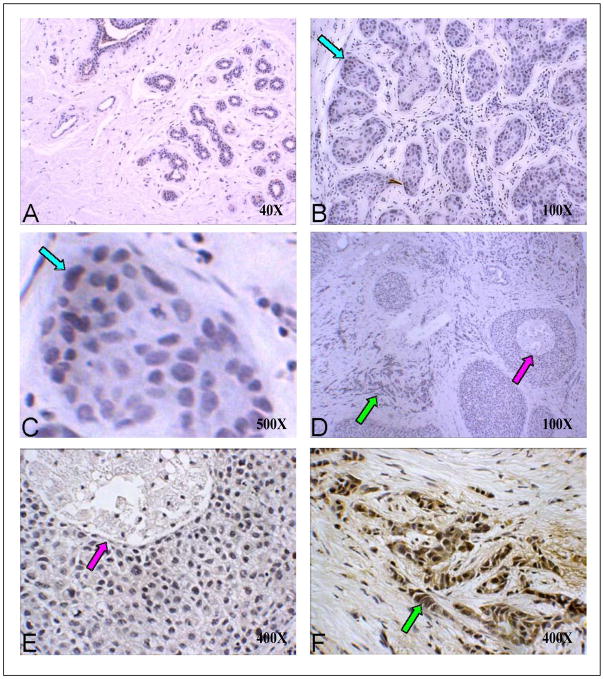
IsoLevuglandins were abundantly present in colon cancer cells lacking normal cell architecture (Figure 2D). In colon cancer, IsoLGs appeared to be present in colon crypts in normal adjacent tissue to the tumor but were not readily detected in cells surrounding the normal colon crypts (Figure 2A). These results suggest that D11 either non-specifically binds to proteins or other molecules within normal colon crypts, or that normal colon crypt may gather or sequester IsoLG-adducted proteins. Trace amounts of IsoLGs were present in cells within normal adjacent tissue to tumors for kidney (Figure 2G), liver (Figure 2M), lung (Figure 3A), pancreas (Figure 3G) and tongue (Figure 3M) specimens. However, elevated concentrations of IsoLGs were found in cells within tumors of the kidney (Figure 2J), liver (Figure 2P), lung (Figure 3D), pancreas (Figure 3J) and tongue (Figure 3P). IsoLGs were not detected in cells from normal breast (Figure 4A), benign hyperplasia or in situ carcinoma (Figures 4B – 4E), but were abundant in invasive breast cancer cells (Figures 4D and 4F). These results suggest that IsoLGs, as represented by D11 scfv staining, are strongly produced in invasive breast cancer.
Figure 4. D11 scfv staining on normal, hyperplasia and malignant breast tissue.
A) Normal breast tissue at low (40X) magnification; B) Breast hyperplasia (100X); C) Benign hyperplasia at high (1,000X) magnification (blue arrows in B and C point to the same site at different magnifications); D) In situ and invasive breast cancer at low (40X) magnification; E) In situ carcinoma at 400X magnification (purple arrows in D and E point to the same site at different magnifications). F) Invasive cancer at 400X magnification (green arrows in D and F point to the same site at different magnifications). Note: only the invasive carcinoma in Panel F shows strong D11 scfv (brown) staining.
Discussion
Isolevuglandins (IsoLGs) are by-products of both the enzymatically derived prostaglandin and the free radical-induced lipid peroxidation pathway resulting from the production of ROS and oxidative stress (Figure 1). Inflammation within tumors can exacerbate oxidative stress and lead to elevated production of free radicals (e.g. ROS) that can induce the formation of IsoLGs via the free radical pathway 24.
The scfv recombinant antibody designated D11 is specific for IsoLGs on proteins or peptides, regardless of amino acid sequence, and has been used in IHC to detect IsoLGs in tissue samples 6, 16.
We hypothesized that ROS produced in tumors leads to increased lipid peroxidation and IsoLG formation. We believed that the concentration of IsoLGs and D11 cell staining in human tumor tissues would be elevated with respect to normal tissue obtained from the same cancer patient. When D11 was used to detect IsoLGs in human tumor and normal adjacent tissue (NAT) to the same tumor, IsoLG concentration appeared to be elevated in tumors of colon, kidney, liver (Figure 2), lung, pancreatic, and tongue (Figure 3). Breast cancer tumor cells were stained by D11 scfv while no staining was visualized within normal breast cells or breast hyperplasia cells (Figure 4), suggesting the prevalence of IsoLGs in tumor cells when compared with their NAT counterparts. In all cases, IsoLG concentrations were greater in human tumor cells in comparison to normal cells in adjacent tissue to the same tumors. These results suggest that oxidative stress and ROS formation, as evidenced by IsoLG formation and D11 IHC staining, occurs in tumor cells but is reduced in cells that are more normal in appearance or distal to the tumors.
It is unclear why IsoLGs were apparently prevalent within normal colon crypts. However, enterocytes line the outside of colon crypts and are capable of peptide, amino acid and lipid uptake. Potentially, enterocytes may also be capable of IsoLG adducted peptide and amino acid uptake in normal tissue adjacent to tumors in which inflammation and ROS production occurs 25. If so, the D11 would stain normal colon crypts in which IsoLG uptake had taken place.
This study was carried out to determine if IsoLGs were present in human tumors as ascertained via IHC using the anti-IsoLG D11 scfv recombinant antibody. The present study was limited in scope, and plasma and urine samples were not obtained from the cancer patients to determine if IsoLG-adducted proteins were also present in plasma and urine. In view of the data presented, a subsequent study - to determine if an increase in IsoLG-adducted proteins in cancer patient plasma or urine coincides with an increase in IsoLG concentration in human tumors - seems intuitive. However, the benefits of such a study using human samples cannot be predicted for the following reasons. IsoLGs rapidly bind to readily available free amines on nearby proteins or nucleic acids. As such, IsoLG binding events may be random and dependent more so upon diffusion rates and the distance between IsoLGs and the free amines on biomolecules. If so, then IsoLG binding may be non-specific; and any attempt to identify unique IsoLG-adducted molecules as biomarkers indicative of a specific disease or stage may not be possible. Before such a study is carried out using human samples, a well-controlled study using murine cancer models would be warranted. The study would be needed to determine if specific IsoLG-adducted proteins or nucleic acids are present in tumors, plasma and urine samples; and if so, are indicative of disease or disease stage. The results of the murine cancer studies would then be used to determine if studies involving humans are justified. We are developing an enzyme-linked immunosorbant assay (ELISA) to capture, detect and quantify IsoLG-adducted proteins. When developed, the ELISA will be used to quantify IsoLG in biological samples, including those obtained from murine and human cancer samples.
The results of the present study showed that a greater abundance of IsoLGs were present in tumor tissues compared to normal adjacent tissue. These results suggest that IsoLGs, stained using D11, may serve as an indicator of oxidative stress in tumor cells. Although isoprostanes or HODEs can serve as markers of lipid peroxidation, IsoLGs adducted proteins are long-lived in comparison and are likely to be more reliable as a gauge of oxidative stress in tumors 26.
Highlights.
The concentration of reactive oxygen species (ROS) in tumor cells can be elevated
ROS can be responsible for lipid peroxidation and isolevuglandin (IsoLG) formation
D11, an antibody specific for IsoLG, was used to detect IsoLG in human tumors
IsoLG concentration was elevated in human tumors in comparison to normal tissue
IsoLG can serve as a biomarker of lipid peroxidation in human tumors
Acknowledgments
Grant support
This work was supported by start-up funds from the Vanderbilt Institute for Chemical Biology to R. Mernaugh, NIEHS P30 ES000267 pilot project grant to R. Mernaugh and J. Roberts and the Vanderbilt University Medical Center Biomedical Research Education and Training (BRET) Program to S. Estes, J. Maeng and B. Parker.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79:123–130. [PMC free article] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16:217–226. 229. discussion 230–212. [PubMed] [Google Scholar]
- 4.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(Suppl 2):S170–180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 5.Van der Paal J, Neyts EC, Verlackt CCW, Bogaerts A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem Sci. 2016;7:489–498. doi: 10.1039/c5sc02311d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mont S, et al. Accumulation of isolevuglandin-modified protein in normal and fibrotic lung. Sci Rep. 2016;6:24919. doi: 10.1038/srep24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo L, et al. Isolevuglandin-modified phosphatidylethanolamine is metabolized by NAPE-hydrolyzing phospholipase D. Journal of lipid research. 2013;54:3151–3157. doi: 10.1194/jlr.M042556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrow JD, et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyer RS, Ghosh S, Salomon RG. Levuglandin E2 crosslinks proteins. Prostaglandins. 1989;37:471–480. doi: 10.1016/0090-6980(89)90096-8. [DOI] [PubMed] [Google Scholar]
- 10.Murthi KK, Friedman LR, Oleinick NL, Salomon RG. Formation of DNA-protein cross-links in mammalian cells by levuglandin E2. Biochemistry. 1993;32:4090–4097. doi: 10.1021/bi00066a034. [DOI] [PubMed] [Google Scholar]
- 11.Salomon RG. Levuglandins and isolevuglandins: stealthy toxins of oxidative injury. Antioxidants & redox signaling. 2005;7:185–201. doi: 10.1089/ars.2005.7.185. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan CB, Matafonova E, Roberts LJ, 2nd, Amarnath V, Davies SS. Isoketals form cytotoxic phosphatidylethanolamine adducts in cells. Journal of lipid research. 2010;51:999–1009. doi: 10.1194/jlr.M001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomon RG, Bi W. Isolevuglandin adducts in disease. Antioxidants & redox signaling. 2015;22:1703–1718. doi: 10.1089/ars.2014.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 15.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nature reviews Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 16.Davies SS, et al. Localization of isoketal adducts in vivo using a single-chain antibody. Free radical biology & medicine. 2004;36:1163–1174. doi: 10.1016/j.freeradbiomed.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Talati M, et al. Oxidant stress modulates murine allergic airway responses. Free radical biology & medicine. 2006;40:1210–1219. doi: 10.1016/j.freeradbiomed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Boyden PA, et al. Potential role of isoketals formed via the isoprostane pathway of lipid peroxidation in ischemic arrhythmias. J Cardiovasc Pharmacol. 2007;50:480–486. doi: 10.1097/FJC.0b013e31815a0564. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda K, et al. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circulation research. 2005;97:1262–1269. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 20.Kirabo A, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126:1607. doi: 10.1172/JCI87425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao L, et al. Renal Denervation Prevents Immune Cell Activation and Renal Inflammation in Angiotensin II-Induced Hypertension. Circulation research. 2015;117:547–557. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Z, et al. Single-chain fragment variable antibody piezoimmunosensors. Analytical Chemistry. 2005;77:797–805. doi: 10.1021/ac048655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 25.Snoeck V, Goddeeris B, Cox E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 2005;7:997–1004. doi: 10.1016/j.micinf.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Poliakov E, et al. Isolevuglandins, a novel class of isoprostenoid derivatives, function as integrated sensors of oxidant stress and are generated by myeloperoxidase in vivo. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:2209–2220. doi: 10.1096/fj.03-0086com. [DOI] [PubMed] [Google Scholar]