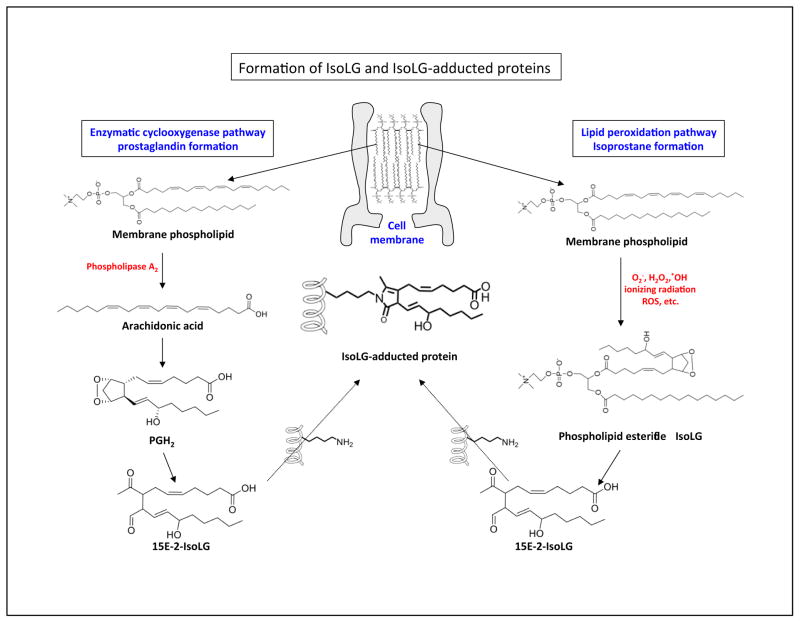
Figure 1. Formation of isolevuglandin (IsoLG) and IsoLG protein adducts.
Two pathways are used to form IsoLG protein adducts. The enzymatically derived prostaglandin pathway (left) and the free radical (lipid peroxidation) derived isoprostane pathway (right) and are depicted. (Left) Phospholipase A2 (PLA2) can cleave phospholipids to form arachidonic acid. Cyclooxygenase can oxidize arachidonic acid to form prostaglandin H2 that can subsequently undergo chemical rearrangement to form IsoLG (e.g. the 15E-2-IsoLG regioisomer). (Right) ROS stemming from mitochondria in a hypoxic tumor microenvironment can lead to lipid peroxidation. Oxidized lipids can undergo non-enzymatic chemical rearrangement to form IsoLG and IsoLG protein adducts. The chemically reactive IsoLG can then adduct to free amines (-NH2) on proteins or DNA (not depicted).