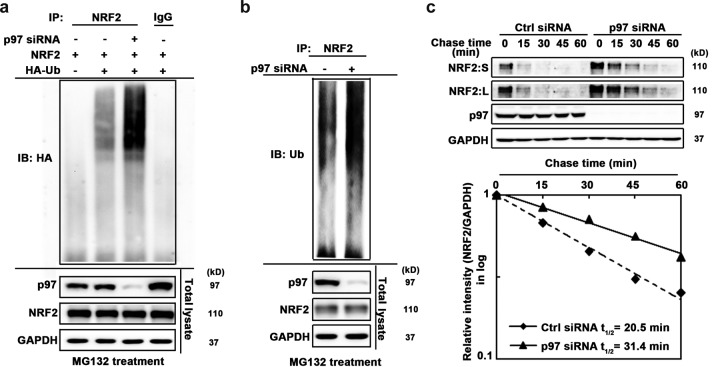
FIG 3.
p97 facilitates ubiquitin-mediated degradation of NRF2. (a) Ubiquitylation of ectopically expressed NRF2. H1299 cells were first transfected with p97 siRNA for 24 h and then transfected with expression vectors for FLAG-NRF2 and HA-ubiquitin (HA-Ub) for an additional 24 h. Before harvest, cells were treated with 10 μM MG132 for 4 h to block degradation of ubiquitylated NRF2. NRF2 was immunoprecipitated using an anti-FLAG antibody, and immunoprecipitated NRF2 was subjected to immunoblot analysis with an anti-HA antibody. (Bottom) An aliquot of total cell lysate was used for immunoblot analysis. (b) Ubiquitylation of endogenous NRF2. H1299 cells were transfected with p97 siRNA for 48 h. NRF2 was immunoprecipitated with an anti-NRF2 antibody, and immunoprecipitated NRF2 was subjected to immunoblot analysis with an antiubiquitin (anti-Ub) antibody for detection of endogenous ubiquitylated NRF2. (Bottom) An aliquot of total cell lysate was used for immunoblot analysis. (c) H1299 cells were transfected with a scrambled siRNA (Ctrl siRNA) or p97 siRNA for 48 h. Cells were treated with cycloheximide for the indicated time before harvest. (Top) Total cell lysates were subjected to immunoblot analysis. (Bottom) NRF2 and GAPDH levels were determined by densitometry, and the level of NRF2 relative to that of GAPDH was plotted as a function of time to determine the half-life of NRF2. NRF2:S, short exposure; NRF2:L, long exposure.