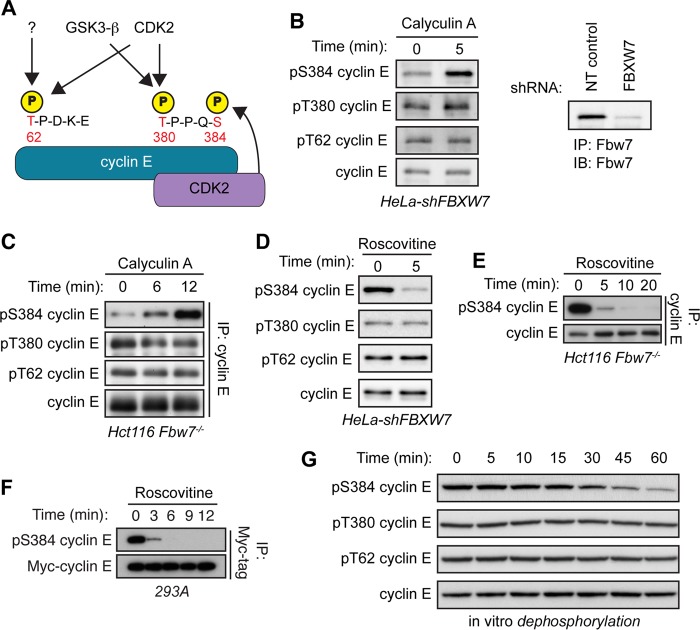
FIG 1.
Cyclin E is dephosphorylated specifically at S384 both in vivo and in vitro. (A) Schematic depicting cyclin E CPDs and the relevant kinases phosphorylating each residue. Cyclin E contains two CPDs: a low-affinity N-terminal degron centered at T62 that contains only one phosphosite (pT62) and a high-affinity C-terminal degron centered at T380 that contains two phosphosites (pT380 and pS384). All three cyclin E CPD phosphosites can be phosphorylated by CDK2. T62 can also be phosphorylated by an unknown kinase, while T380 can also phosphorylated by glycogen synthase kinase 3-β (GSK3-β). Importantly, S384 can only be phosphorylated by CDK2 in cis (by the bound CDK2 molecule), as depicted. (B) HeLa cells stably expressing an shRNA against FBXW7 (HeLa-shFBXW7) were treated with the serine/threonine phosphatase inhibitor calyculin A for 5 min. Endogenous cyclin E was immunoprecipitated from whole-cell lysates, and changes in phosphorylation of T62, T380, and S384 (all three cyclin E CPD phosphosites) were determined by Western blotting (IB). Efficiency of Fbw7 knockdown was determined by analyzing Fbw7 protein depletion in HeLa cells transduced with either nontargeting control shRNA (NT control) or FBXW7-specific shRNA. (C) Hct116 Fbw7−/− cells were treated with calyculin A for up to 12 min and processed as for panel B. (D) HeLa-shFBXW7 cells were treated with the CDK1/2 inhibitor roscovitine for 5 min and processed as for panel B. (E) Hct116 Fbw7−/− cells were treated with roscovitine for up to 20 min and processed as for panel B. (F) 293A cells were cotransfected with Myc-cyclin E and HA-CDK2 and then treated with roscovitine for up to 12 min. Myc-cyclin E was immunoprecipitated from whole-cell lysates, and changes in S384 phosphorylation were assayed by Western blotting. (G) 293A cells were lysed in buffer lacking phosphatase inhibitors and incubated with recombinant cyclin E for 0 to 60 min in an in vitro dephosphorylation assay. Changes in CPD dephosphorylation over time were measured by Western blotting.