FIG 3.
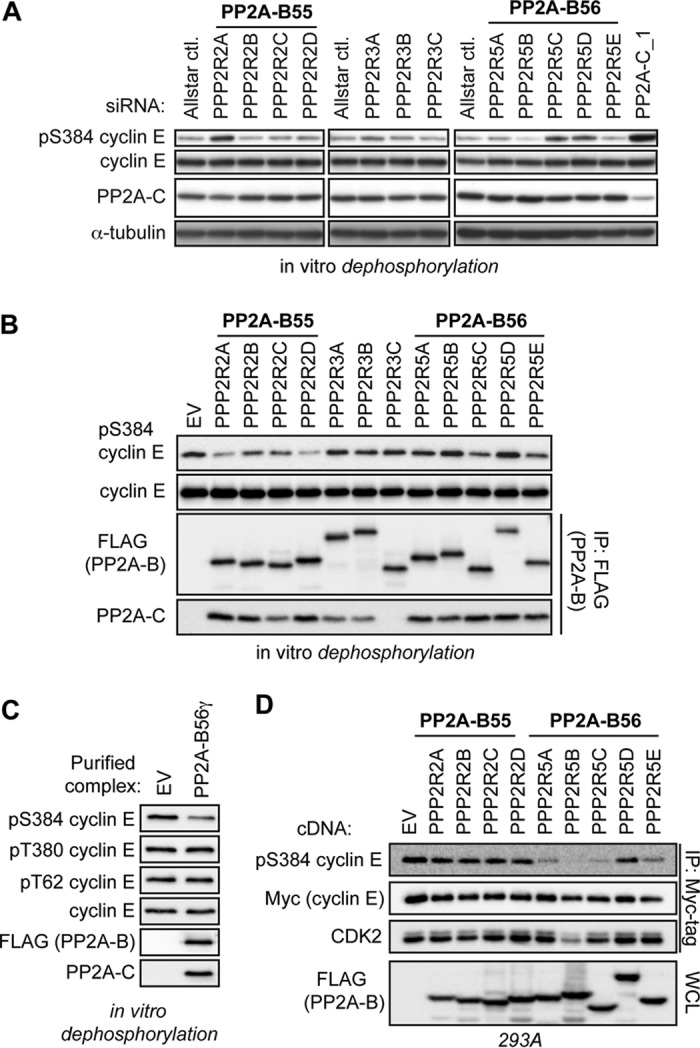
PP2A-B56 complexes regulate cyclin E S384 phosphorylation in vitro and in vivo. (A) Specific PP2A B subunits were depleted by transfecting 293A cells with pools of siRNAs, as done for Fig. 2B. Lysates harvested from these cells were incubated with purified cyclin E-CDK2 complexes in an in vitro dephosphorylation assay. Lysates depleted of the PP2A catalytic subunit were used as a positive control (siPP2A-C_1, far right lane). (B) 293A cells were transfected with FLAG-B subunits and then lysed in Tween 20 lysis buffer without phosphatase inhibitors. PP2A holoenzymes were purified via anti-FLAG immunoprecipitation and then incubated with recombinant cyclin E-CDK2 complexes to test for direct dephosphorylation of S384 in vitro. IPs from cells transfected with an empty vector (EV) served to control for nonspecific coprecipitating phosphatase activity. The relative abundance of PP2A-C served to approximate the efficiency of purification of intact PP2A heterotrimers. (C) 293A cells were transfected with either an EV or FLAG-tagged B56γ and lysed in Tween 20 lysis buffer without phosphatase inhibitors. Next, anti-FLAG immunoprecipitations were used to purify PP2A-B56γ complexes; immunoprecipitations of EV lysate served as a control for nonspecific background phosphatase activity. These complexes were then used for in vitro dephosphorylation reactions of recombinant cyclin E-CDK2. The specificity of PP2A-B56γ for each cyclin E CPD phosphosite was determined by Western blotting. (D) 293A cells were cotransfected with FLAG-B subunits, Myc-cyclin E, and HA-CDK2. Cells were lysed, and exogenous cyclin E was immunoprecipitated and analyzed for pS384 and bound CDK2 by Western blotting.
