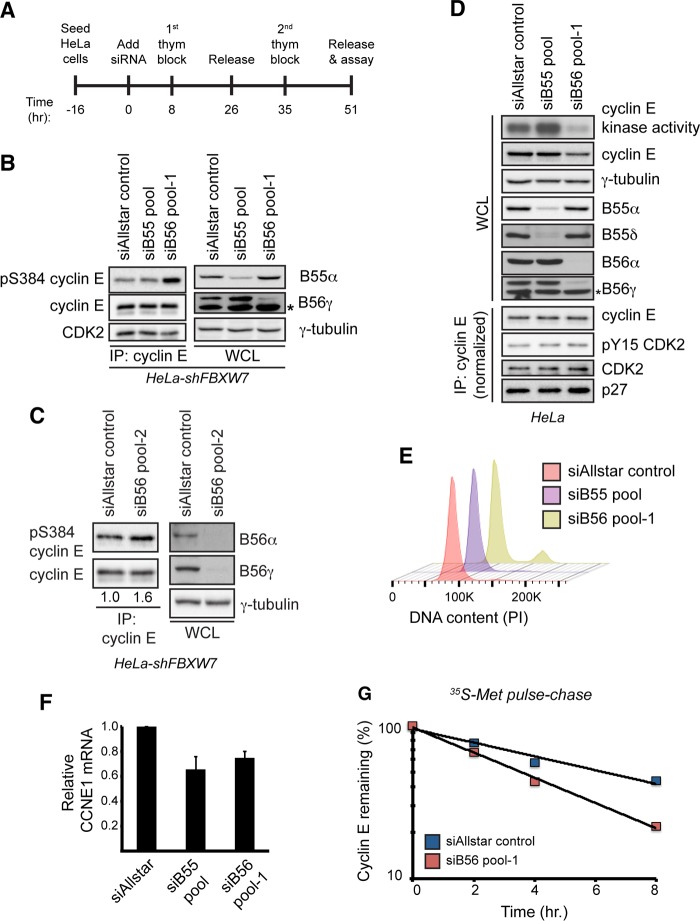
FIG 4.
PP2A-B56 controls cyclin E kinase activity and protein stability. (A) Schematic of the experimental protocol used for the other panels. Cells were seeded and transfected with siRNAs on the next day (time zero). Cells were then synchronized at the G1/S transition using a double-thymidine block, and endpoint assays were conducted approximately 51 h after siRNA transfection. (B) HeLa-shFBXW7 cells were transfected with siRNAs targeting all four PP2A-B55 regulatory subunits (siB55 pool), all five PP2A-B56 regulatory subunits (siB56 pool 1), or a negative-control siRNA (siAllstar control). Following transfection, the cells were synchronized as depicted in panel A, and changes in S384 phosphorylation were assayed by Western blotting. To measure siRNA efficacy, the abundances of endogenous B55α and B56γ isoforms were analyzed by Western blotting. The asterisk indicates a nonspecific band that cross-reacts with the B56γ antibody. (C) HeLa-shFBXW7 cells were transfected with an independent set of siRNAs targeting all five PP2A-B56 regulatory subunits (siB56 pool 2) or a negative-control siRNA (siAllstar control) and processed as described for panel B. Numbers correspond to relative cyclin E S384 phosphorylation normalized to total immunoprecipitated cyclin E. (D) HeLa cells were transfected with siRNAs targeting all four PP2A-B55 regulatory subunits (siB55 pool), all five PP2A-B56 regulatory subunits (siB56 pool 1), or a negative-control siRNA (siAllstar control). Following transfection, cells were synchronized as depicted in panel A, released for 2 h, and assayed for cyclin E protein abundance and kinase activity. To assay for changes in the amount of bound CDK2, CDK2 inhibitory phosphorylation, and bound p27, we performed cyclin E immunoprecipitations after normalizing the input for the amount of cyclin E present in the whole-cell extract. To measure siRNA efficacy, the abundances of multiple B55 (B55α and B55δ) and B56 (B56α and B56γ) isoforms were analyzed by Western blotting. An asterisk indicates a nonspecific band that cross-reacts with the B56γ antibody. (E) Cell cycle fluorescence-activated cell sorter (FACS) analysis of samples treated as for panel D. DNA was stained using propidium iodide (PI). (F) Analysis of CCNE1 transcript levels in cells treated as for panel D. Transcripts were normalized to ACTB expression. Data are means ± SEMs of two independent biological replicates. (G) Endogenous cyclin E half-life was measured using a [35S]Met pulse-chase in cells synchronized at G1/S, as depicted in panel A.