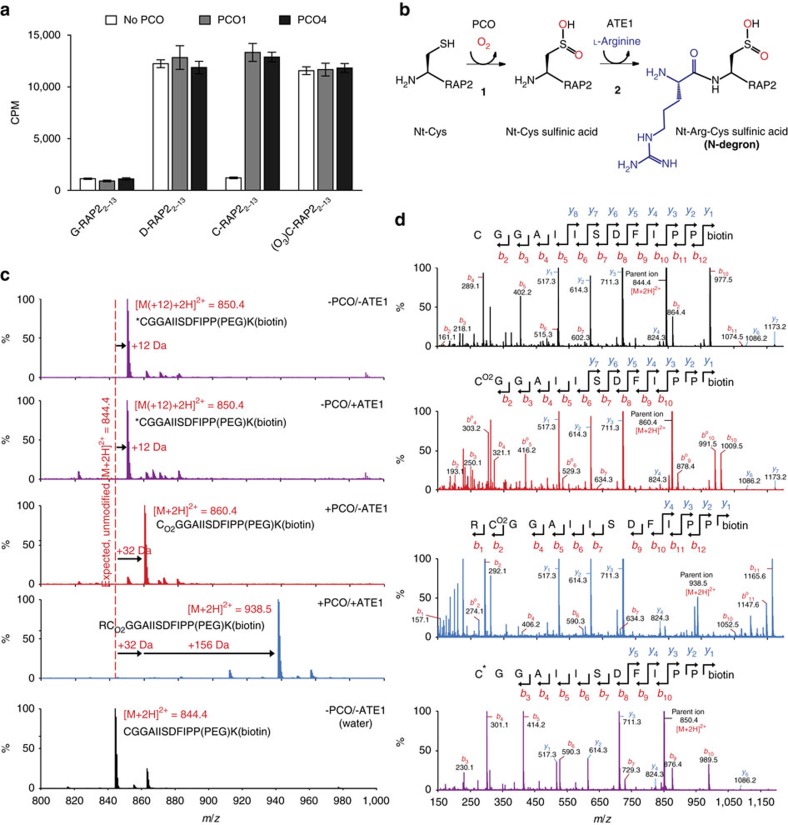
Figure 4. PCO-catalysed Cys-sulfinic acid formation renders RAP22–13 a substrate for ATE1-catalysed arginylation.
(a) 14C-Arg incorporation by ATE1 into the 12-mer N-terminal ERF-VII peptide (H2N-XGGAIISDFIPP(PEG)K(biotin)-NH2, X=Gly, Asp, Cys or Cys-sulfonic acid (C(O3))), was assayed by liquid scintillation counting of immobilized biotinylated peptides after the arginylation reaction and removal of unreacted 14C-Arg (n=3). In the case of the Cys-starting peptide (RAP22–13), ATE1 activity was strongly dependent on the presence of PCO1 or PCO4. n=3, error bars in this panel represent s.e.m. (b) Scheme showing PCO- and ATE1-catalysed reactions on Nt-Cys of ERF-VIIs, as validated in this study. (c) LC–MS spectra of products of equivalent assays with Cys-initiated RAP22–13 using non-radiolabelled Arg, revealing a sequential mass increase of +32 (corresponding to oxidation) and +156 Da (corresponding to arginylation) only in the presence of PCO and ATE1 (blue spectrum). The red spectrum shows a +32 Da mass increase for Cys-RAP22–13 incubated +PCO/−ATE, demonstrating Cys-sulfinic acid formation as expected. Purple spectra show +12 Da species formed upon incubation of Cys-RAP22–13 in the absence of PCO+/−ATE (for explanation of this mass increase see text and Supplementary Fig. 6); the black spectrum shows Cys-RAP22–13 dissolved in H2O. (d) b- and y-ion series spectra generated by MS/MS analysis of Cys-RAP22–13 only (no incubation; black), Cys-RAP22–13 incubated +PCO/−ATE (red), Cys-RAP22–13 incubated +PCO/+ ATE1 (blue) and Cys-RAP22–13 incubated −PCO/− ATE1 (purple), confirming arginylation only at the N terminus of PCO-modified RAP22–13.