-
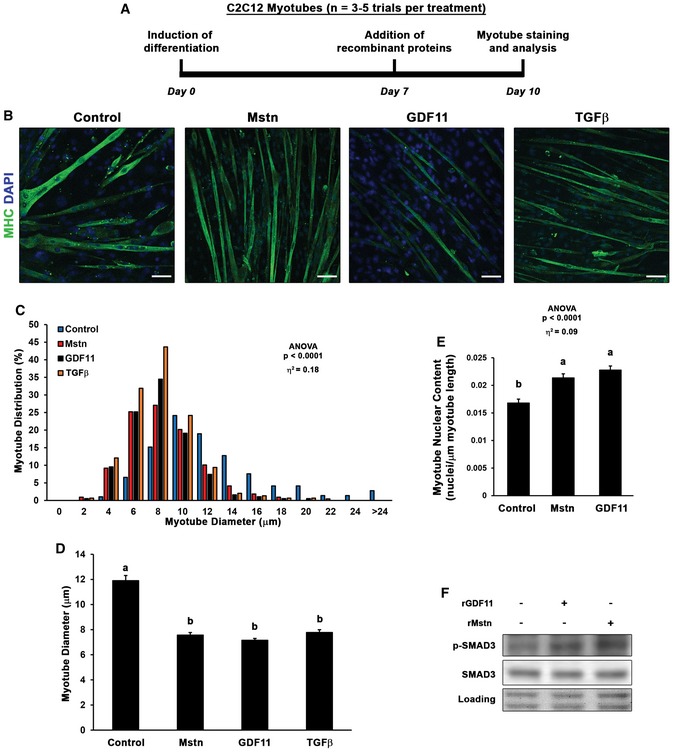
A, B
C2C12 myoblasts were differentiated for seven days, then control (n = 5), myostatin (Mstn; n = 4), growth differentiation factor 11 (GDF11; n = 5), or transforming growth factor β (TGFβ; n = 3) supplemented media (50 ng/ml) was added for 3 days (depicted in A) before PFA fixation and staining with myosin heavy chain (MHC) to evaluate myotube diameter (B). The scale bars represent 50 μm.
-
C, D
Myotube diameter data (n = 200–400 myotubes from 3 to 5 different trials) are depicted as a histogram of diameter distribution (C) and as mean diameter (mean ± SEM; D) by treatment.
-
E
Myotube nuclear content was quantified as DAPI‐positive nuclei per μm of myotube length for control, Mstn, and GDF11 treatment groups.
-
F
Phosphorylation of SMAD3 in control, Mstn, and GDF11 treatment groups, as detected by immunoblotting. Equal loading is verified by Ponceau Red staining.
Data information: Data are depicted as histogram of distribution (C) or mean ± SEM (D, E). Statistical analysis was performed using one‐way ANOVA analysis with Tukey's HSD
post hoc test (non‐connecting letters indicate
P < 0.05 between groups) and effect size presented as eta‐squared (η
2).