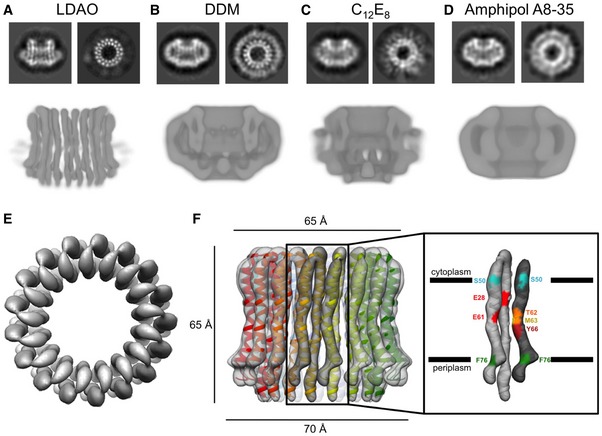
Figure 4. Electron cryo‐microscopy of the Burkholderia pseudomallei N‐type ATPase rotor ring.
-
A–DRepresentative 2D class averages of the rotor ring showing the ring from the side and top as well as slices through 3D maps after refinement for (A) LDAO, (B) DDM, (C) C12E8, and (D) amphipol A8‐35.
-
ETop view of the rotor ring in LDAO with 17 identical hairpin‐like c‐subunits fitted.
-
FSide view of the c‐ring in LDAO with the refined model fitted. The helices in the outer ring are slightly shorter than the inner helices. Two adjacent c‐subunits form a functional ion‐binding unit. The positions of residues involved in ion binding or defining the membrane boundary are shown in different colors.
