Figure 5. EDD‐DYRK2‐DDB1Vpr BP‐mediated ubiquitination and degradation of CP110 are regulated by Cep78.
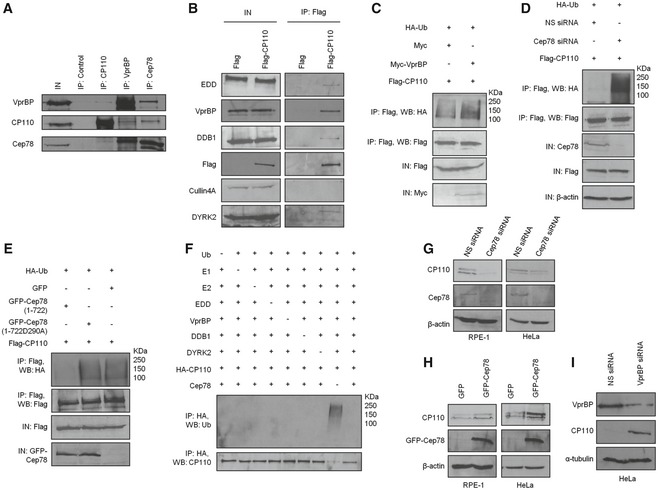
- HEK293 lysates were immunoprecipitated with an irrelevant (control), anti‐CP110, anti‐VprBP or anti‐Cep78 antibody, and Western blotted with the indicated antibodies. IN, input.
- Flag or Flag‐CP110 was expressed in HEK293 cells. Lysates were immunoprecipitated with an anti‐Flag antibody and Western blotted with the indicated antibodies. IN, input.
- Flag‐CP110 was co‐expressed with HA‐Ub and Myc or Myc‐VprBP in HEK293 cells. Lysates were immunoprecipitated with an anti‐Flag antibody in 1% SDS and Western blotted with the indicated antibodies. IN, input.
- HEK293 cells were transfected with NS siRNA or Cep78 siRNA and constructs expressing Flag‐CP110 and HA‐Ub. Lysates were immunoprecipitated with an anti‐Flag antibody in 1% SDS and Western blotted with the indicated antibodies. IN, input. β‐actin was used as a loading control.
- Flag‐CP110 was co‐expressed with HA‐Ub and GFP, GFP‐tagged Cep78 wild type (1–722), or mutant refractory to VprBP binding (1‐722D290A) in HEK293 cells. Lysates were immunoprecipitated with an anti‐Flag antibody in 1% SDS and Western blotted with the indicated antibodies. IN, input.
- In vitro ubiquitination assays were performed with HA‐CP110 as a substrate in the presence of purified Ub, E1, E2, EDD, DYRK2, DDB1, VprBP, and Cep78 in various combinations. Ubiquitinated and non‐ubiquitinated CP110 were detected by immunoblotting with anti‐Ub and anti‐CP110 antibodies, respectively.
- RPE‐1 or HeLa cells were transfected with NS siRNA or Cep78 siRNA. Lysates were Western blotted with the indicated antibodies. β‐actin was used as a loading control.
- RPE‐1 or HeLa cells were transfected with construct expressing GFP or GFP‐Cep78. Lysates were Western blotted with the indicated antibodies. β‐actin was used as a loading control.
- HeLa cells were transfected with NS siRNA or VprBP siRNA. Lysates were Western blotted with the indicated antibodies. α‐tubulin was used as a loading control.
Source data are available online for this figure.
