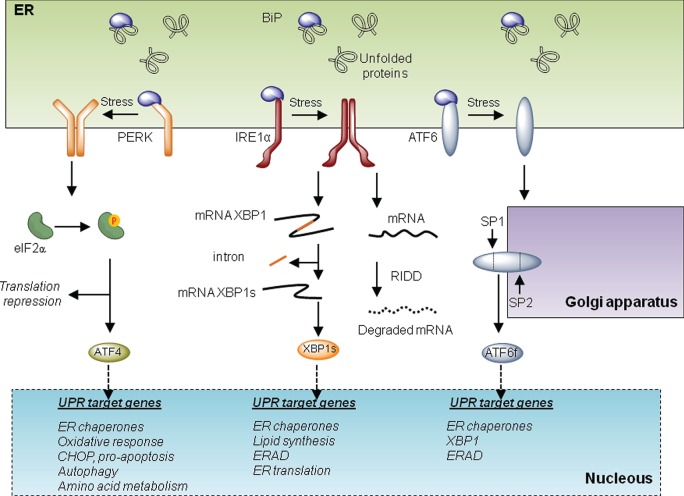
Figure 1.
The unfolded protein response (UPR). Three main signaling branches form the UPR. Under resting conditions, BiP protein binds to and inhibits the triggering of the UPR. Under endoplasmic reticulum (ER) stress, BiP dissociates from the UPR transducers to chaperone misfolded proteins in the lumen of the ER. This disassemble promotes the activation of the three branches of the UPR. On the one hand, PKR-like ER kinase (PERK) oligomerizes and phosphorylates eIF2alpha. This phosphorylation provokes a repression of global translation and facilitates the expression of specific transcripts. Among them, activating transcription factor 4 (ATF4) drives the transcription program of the PERK branch that activates genes involved in folding, oxidative responses, autophagy, amino acid metabolism, and apoptosis via CHOP. Upon activation, IRE1 oligomerizes and processes the mRNA encoding for X-box binding protein 1 (XBP1), a transcription factor that activates cellular programs involved in ERAD, ER translation, ER chaperones, and lipid synthesis. Finally under stress, activating factor 6 (ATF6) translocates to the Golgi where it is processed by SP1 and SP2 generating a transcription factor that activates UPR target genes involved in ERAD and folding.