Figure 1.
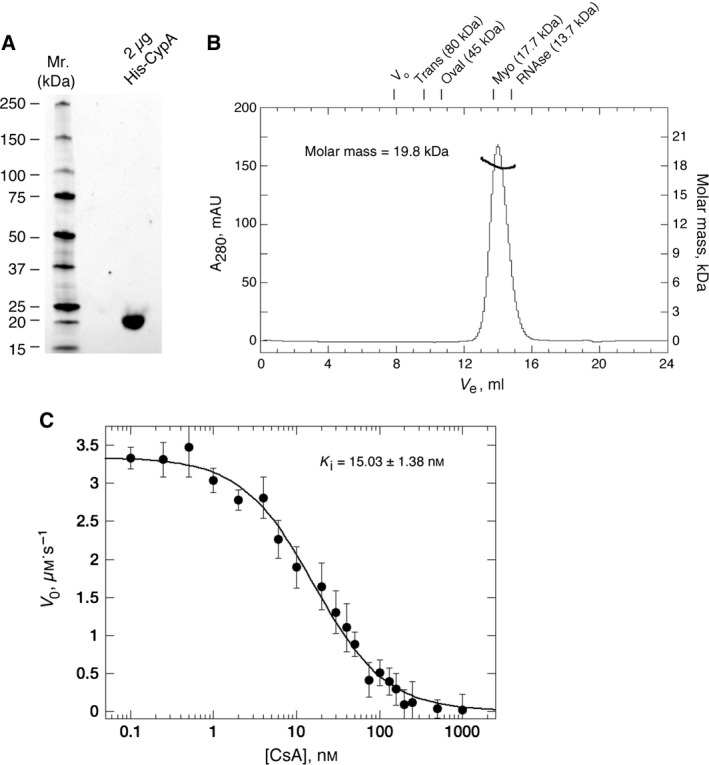
(A) Ultrapure, monodisperse and highly active protein is used for sensor‐surface generation. (A) 4–15% acrylamide SDS Stain‐free TGX gel (BioRad, Hercules, CA, USA) illustrating the final purity of His‐CypA (2 μg total protein). Standards are shown to the left. (B) Size‐exclusion chromatography (ÅKTA‐Micro; GE Healthcare) coupled with UV, static light scattering and RI detection (Viscotec SEC‐MALS 20 and Viscotek RI Detector:VE3580; Malvern Instruments), were used to determine the molar mass of His‐CypA in solution. About 100 μL of 1 mg·mL−1 His‐CypA was run on a Superdex‐75 10/300 GL (GE Healthcare) size exclusion column pre‐equilibrated in 10 mm NaH2 PO 4, pH 7.5; 150 mm NaCl at 22 °C, at 0.8 mL·min−1. Light scattering, RI and A 280 nm were analysed by a homo‐polymer model (omnisec software, v 5.1; Malvern Instruments) using the following parameters: ∂A/∂c at 280 nm 0.71 AU·mL−1·mg−1 and ∂n/∂c of 0.185 mL·g−1. His‐CypA protein elutes a single sharp peak with a correlative R s of 1.82 ± 0.1 nm (mean ± SEM, n = 5). Elution positions for standards are shown above the chromatograph. The molar mass average across the elution profile is 19.8 kDa with excellent monodispersity (Mw/Mn = 1.003). The theoretical molecular weight of His‐CypA is 21.04 kDa. DLS analysis (data not shown) also indicates high monodispersity for His‐CypA solutions with a mean R h of 1.85 ± 0.17 nm (mean ± SEM, n = 5), a polydispersity index of ≤ 0.1, and a correlative molecular weight of between 19 and 21 kDa, consistent with a highly pure and monomeric protein solution. (C) Inhibition of His‐CypA's PPIase activity by CsA. Initial background corrected reaction (V 0) rate in μm·s−1 is plotted versus the concentration of CsA in nm. Solid lines are a least squares fit of the data to Eqn (1) (see Material and methods). Each point is the mean ± SE, n = 3. The mean K i value for CsA, at 12 °C, is 15.03 ± 1.38 nm.
