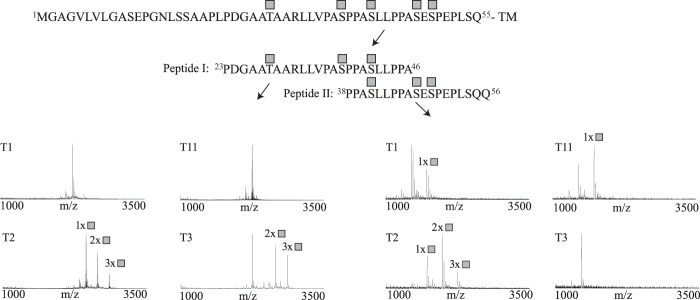
FIGURE 1.
In vitro analysis of GalNAc-T isoform specificity and a summary of detected glycosylation sites of hβ1AR. Shown is in vitro O-glycosylation analysis with GalNAc-T1, T2, T3, T4, T5, T11, T12, T13, T14, and T16 of two overlapping peptides (peptides I and II) covering the β1AR N terminus. Reactions were followed by MALDI-TOF-MS, and spectra are shown for a 24-h incubation time point for T1, T2, T3, and T11. GalNAc-T2 was found to be the most efficient at glycosylating the two peptides, with a total of five sites corresponding to Thr-28, Ser-37, Ser-41, Ser-47, and Ser-49 in the full-length protein. GalNAc-T3 modified three sites at Thr-28, Ser-37, and Ser-41; GalNAc-T1 and GalNAc-T11 modified one position each (not determined). The spectra show relative intensity. The complete hβ1AR N-terminal sequence and peptide sequences are shown above the spectra. The locations of the attached GalNAcs are shown as gray squares. The results are representative of three independent experiments. TM, transmembrane domain.