FIGURE 7.
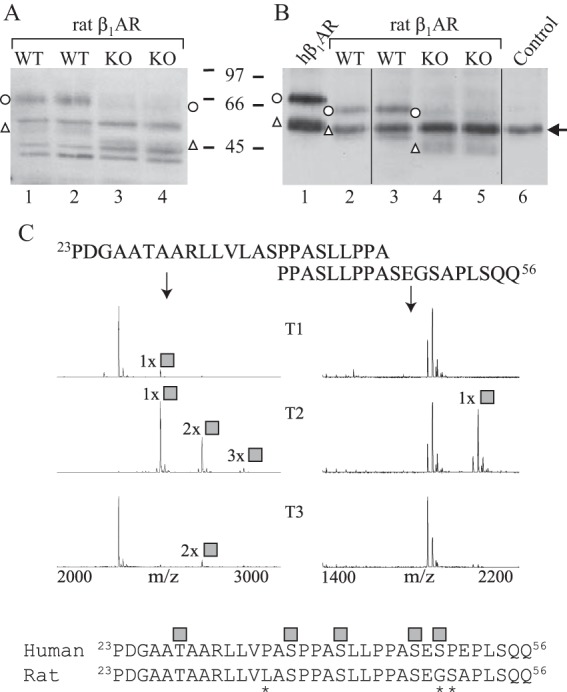
β1AR is cleaved extensively in Galnt2−/− rat hearts. A and B, cellular membranes were isolated from heart ventricles of Galnt2−/− and corresponding WT rats, and receptors were analyzed by SDS-PAGE and Western blotting with an antibody directed against the receptor C-terminal domain (sc-568). The analysis was performed either directly after solubilization (A) or after immunoprecipitation (B). In B, hβ1AR stably expressed in HEK293i cells was used as a control (lane 1). No sample was included for immunoprecipitation for lane 6. Arrow, antibody heavy chain. Open circles, full-length hβ1ARs; open triangles, C-terminal fragments cleaved at 31R↓L32. The experiment was replicated twice using a total of three Galnt2−/− and three WT rat hearts. C, overlapping peptides covering amino acid positions 23–46 of the rat β1AR N terminus were tested for glycosylation by 10 recombinant GalNAc-Ts and analyzed by MALDI-TOF. The 24-h incubation time points are shown. GalNAc-T2 was found to be the most efficient in glycosylating the peptides, whereas GalNAc-T1 and GalNAc-T3 were found to glycosylate the peptide 23PDGAATAARLLVLASPPASLLPPA46 to a minor extent. Shown below is the alignment of the human and rat β1AR N termini; asterisks denote differences in sequence. The identified O-glycosylation sites of the human receptor are shown as gray squares. The spectra show relative intensity. The results shown are representative of two independent experiments.
