FIGURE 6.
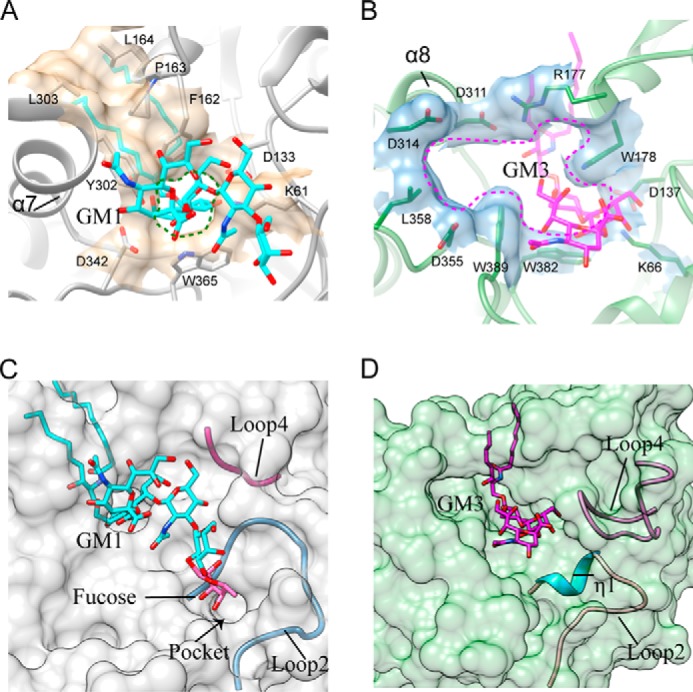
Comparison of the active site pockets of 103S_EGCase I and M777_EGCase II. A, Phe162, Pro163, Leu164, Tyr302, and Leu303 in 103S_EGCase I form a large cap over the active site. Lys61, Asp133, Phe162, Tyr302, Asp342, and Trp365 form a small opening for the active site pocket. B, Arg177 and Asp311 in M777_EGCase II form a small cap over the active site. Lys66, Asp137, Arg177, Trp178, Asp311, Asp314, Asp355, Leu358, Trp382, and Trp389 in M777_EGCase II form a large opening for the active site pocket. C, loop 2 and the short loop 4 of 103S_EGCase I define a broad sugar-binding cavity. The model of fucosyl-GM1 in the binding site was created by superimposing its structure onto GM1 and then adjusting the fucosyl unit to a reasonable conformation using Coot. The small pocket was able to accommodate the fucosyl unit. D, loop 2 and the long loop 4 of M777_EGCase II define a crowded sugar-binding cavity.
