FIGURE 1.
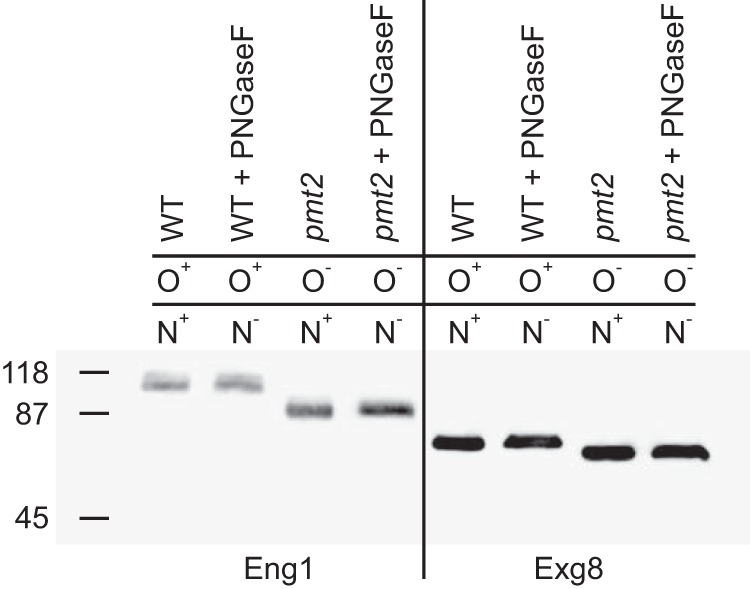
Eng1 and Exg8 have O-linked but not N-linked glycans. Glycan attachment to Eng1 and Exg8 proteins was determined by increased electrophoretic mobility of proteins when O-linked or N-linked glycans were absent. Culture filtrates from Histoplasma expressing Eng1-FLAG- or Exg8-FLAG-tagged proteins were prepared, and the Eng1 and Exg8 proteins were detected by immunoblotting for the FLAG epitope following electrophoretic separation. To prevent O-linked glycan attachment, fusion proteins were expressed in a mutant lacking O-linked mannosylation (pmt2) instead of wild type (WT). N-Linked glycans were removed by treatment of culture filtrate proteins with PNGaseF. The prevention of O-linked glycan attachment or removal of N-linked glycans are indicated by “−” in contrast to normal presence of the glycans (“+”).The molecular mass (kDa) of protein standards is indicated on the left of the immunoblot.
