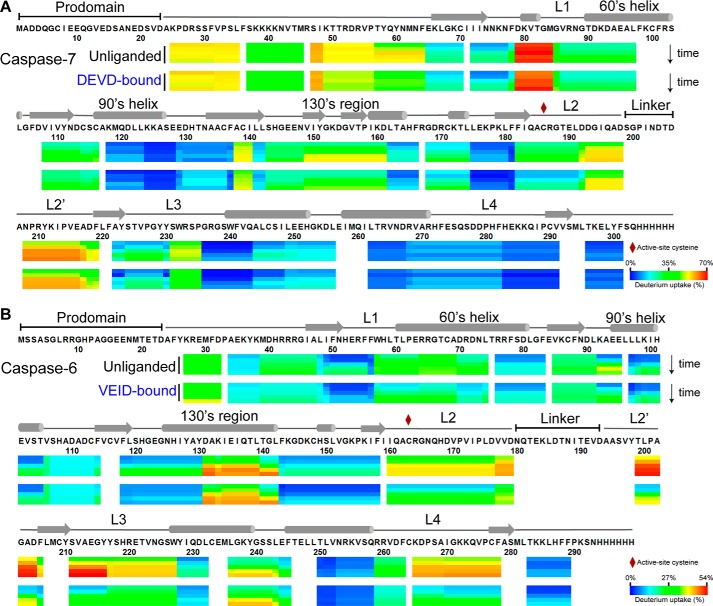
FIGURE 2.
H/D exchange heat map of the relative deuterium incorporation. For each peptic peptide of the unliganded and the peptide-based substrate mimic-bound states of caspase-7 (A) and caspase-6 (B), the percentage relative deuterium level for each H/D exchange incubation time (0.17, 1, 10, 60, and 120 min) is mapped onto its corresponding linear sequence. The percentage relative deuterium incorporation is calculated by dividing the observed deuterium uptake by the theoretical maximum deuterium uptake for each peptide. The H/DX-MS experiments followed 64 peptides common to both unliganded and DEVD-bound caspase-7 that covers 93% of the linear sequence. Likewise, H/DX-MS experiments followed 70 peptides common to both unliganded and VEID-bound caspase-6 that covers 91% of the linear sequence. Peptic peptides with no H/D exchange data at any given incubation time are colored white. All caspase-6 and caspase-7 variants used in the H/DX-MS experiments were cleaved, active forms lacking both the prodomain (residues 1–23 in both caspase-6 and caspase-7) and linker (residues 180–193 in caspase-6; residues 199–206 in caspase-7). The secondary structural elements are also shown above the caspase-6 and caspase-7 sequences. The percentage relative deuterium level of each peptic peptide represents the average values of duplicate experiments performed on two separate days.