FIGURE 5.
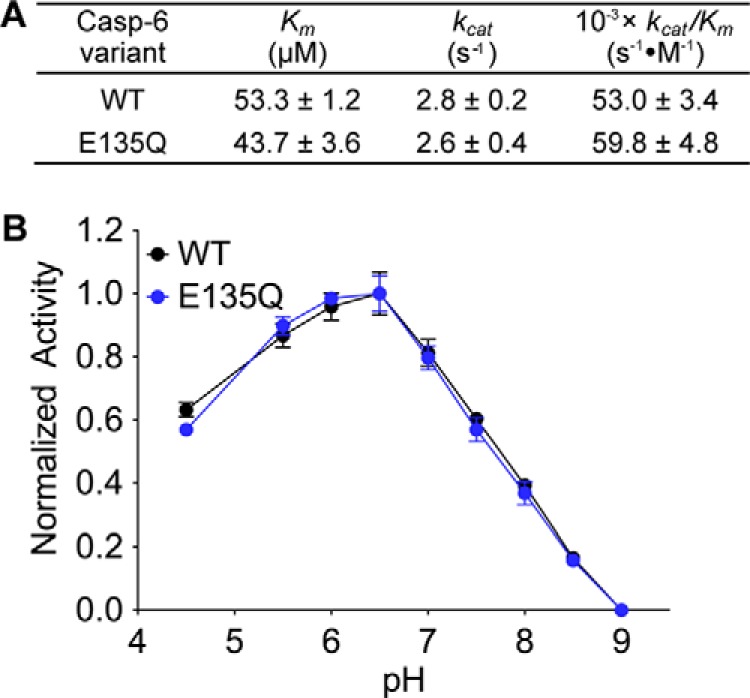
E135Q has kinetics and pH profile similar to those of WT caspase-6. A, the Michaelis-Menten kinetic parameters of caspase-6 WT and the “constantly protonated” variant, E135Q. The values are reported as mean ± S.E. of three independent trials performed on three separate days. B, the activity of caspase-6 WT (black line) and E135Q (blue line) variants as a function of pH. For normalization, the highest and the lowest relative fluorescence response for each data set was set to 100 and 0%, respectively, and reported as fractions. Error bars, S.D. of duplicate measurements on two separate days. All caspase-6 activity assays used fluorescence-based measurements following cleavage of a peptide-based substrate mimic, VEID-AMC, by caspase-6.
