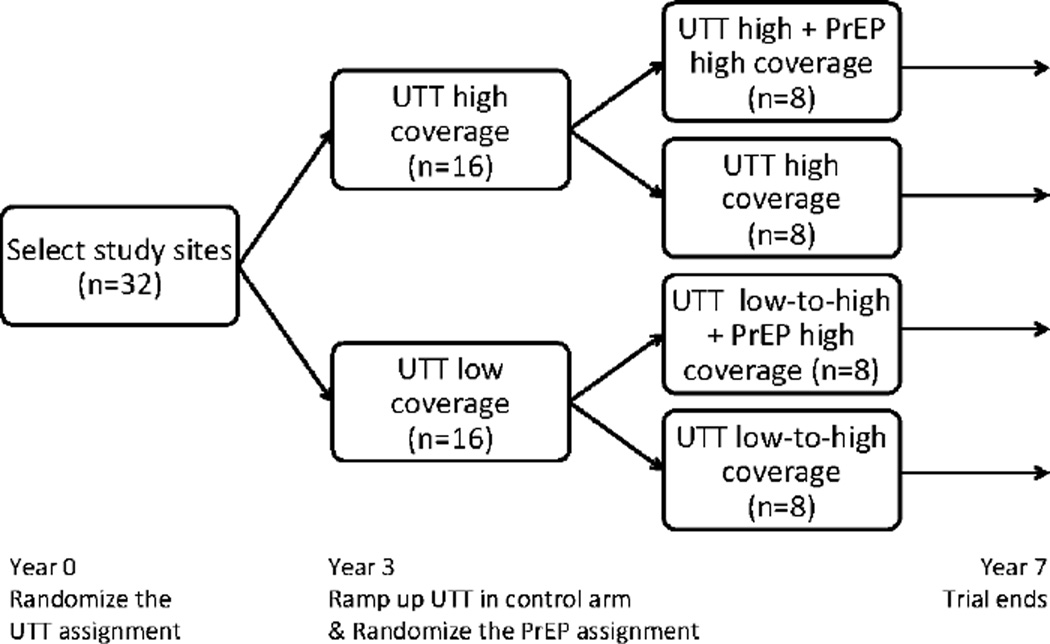
Figure 2.
Proposed Design 2: The universal test-and-treat (UTT) assignment (high vs. lower coverage) is randomized with balanced allocation to the 32 villages. After three years of follow-up, the coverage of antiretroviral therapy is scaled-up in the UTT control arm (from low to high coverage), and the PrEP assignment (high vs. no coverage) is randomized within both UTT arms separately. All villages are followed for four additional years after the second randomization.