Abstract
Purpose:
Our current study was conducted to identify patients’ anatomic, pathologic, and clinical factors to predict difficulty of performing laparoscopic abdominoperineal resection for ultra-low rectal cancer.
Materials and Methods:
Records of 117 consecutive patients with rectal cancer 2 to 5 cm from the anal verge were retrospectively reviewed. Using univariate and multivariate linear or logistic regression models, standardized operative time and blood loss, as well as postoperative morbidity were utilized as endpoints to screen patients’ multiple variables to predict operative difficulty.
Results:
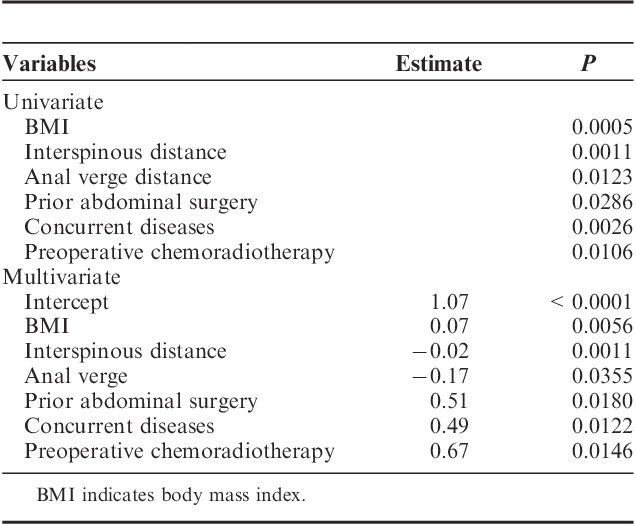
Multivariate linear regression analysis showed body mass index (BMI) (estimate=0.07, P=0.0056), interspinous distance (estimate=−0.02, P=0.0011), tumor distance from anal verge (estimate=−0.17, P=0.0355), prior abdominal surgery (estimate=0.51, P=0.0180), preoperative chemoradiotherapy (estimate=0.67, P=0.0146), and concurrent diseases (hypertension and/or diabetes mellitus) (estimate=0.49, P=0.0122) are predictors for standardized operative time. Age (estimate=0.02, P=0.0208) and concurrent diseases (estimate=0.43, P=0.0476) were factors related to standardized blood loss. BMI (estimate=0.15, P=0.0472) was the only predictor for postoperative morbidity based on logistic regression analysis.
Conclusions:
Age, BMI, interspinous distance, tumor distance from anal verge, prior abdominal surgery, preoperative chemoradiotherapy, and concurrent diseases influence the difficulty of performing laparoscopic abdominoperineal resection for ultra-low rectal cancer. Standardized operative time allows researchers to amass samples by pooling data from all published studies, thus building reliable models to predict operative difficulty for clinical use.
Key Words: laparoscopic surgery, abdominoperineal resection (APR), rectal cancer, operative difficulty
Laparoscopic surgery for colorectal cancer is now widely accepted as a safe and less invasive alternative to open surgery. Compared with open surgery, it has similar short1 and long-term2–5 outcomes, but with more clinical advantages, such as less blood loss and postoperative pain, faster recovery, and less hospital stay time.1,6,7 Laparoscopic surgery enables the shortening of time to initiate chemotherapy in colorectal cancer patients following surgery.8
Unlike the upper abdomen, pelvic space is not expandable by the pneumoperitoneum. Laparoscopic resection for rectal cancer is performed in the pelvis with limited working space. It is therefore a surgery demanding a high skill. Patient’s anatomic, pathologic, and clinical factors have been identified to influence the operative difficulty.9–14 It is observed that most of these previous studies focus on laparoscopic sphincter preserving resection of rectal cancer. Results from these studies are still inconsistent. Limited sample sizes and inclusion of confounding factors, such as surgeons of different skills and different operative procedures, may be the cause of the inconsistencies. To date, very few studies examine patients’ factors influencing the difficulty of laparoscopic abdominoperineal resection (APR) of rectal cancer.15
The objective of our current study was to identify the predicting role of patients’ anatomic, pathologic, and clinical factors that may influence operative difficulty. Operative time, blood loss, and postoperative morbidity would be applied as endpoints to estimate operative difficulty. Only patients who had rectal cancer 2 to 5 cm from anal verge and underwent laparoscopic APR were included. All surgeries were performed by 1 experienced surgeon at the same hospital.
MATERIALS AND METHODS
Patient Selection
From April 2009 to December 2014, a total of 117 consecutive patients with rectal cancer (2 to 5 cm from the anal verge) underwent laparoscopic APR. All surgeries were performed by an experienced surgeon (W.C.). Because of the retrospective nature of the study, informed consent was waived by the Ethics Committee of our hospital.
The following patients’ information were collected from the medical records: age, sex, body mass index (BMI), pelvic dimension, prior abdominal surgery, concurrent diseases (hypertension and/or diabetes mellitus), preoperative chemoradiotherapy, operative time, amount of blood loss during surgery, postoperative morbidity, and duration of hospital stay after surgery, tumor sizes and stagings, and number of lymph nodes harvested. Tumor distance from anal verge was measured by colonoscopy combined with digital examination. Interspinous distance (the narrowest distance between the ischial spines) and sacral-pubis distance (the distance from pubis symphysis to the sacrum at the level of ischial spines) (Fig. 1) were blindly measured on axial computed tomographic images by a radiologist (L.T.). Postoperative pathologic results were used to provide a precise description of the tumors (diameter, the degree of circumferential occupation, and stages). Tumors were staged based upon the seventh tumor node metastasis (TNM) classification of the Union for International Cancer Control (UICC). Operative time, starting from establishment of the pneumoperitoneum, to the end of the abdominal incision sutured, was calculated using electronic anesthesia records.
FIGURE 1.
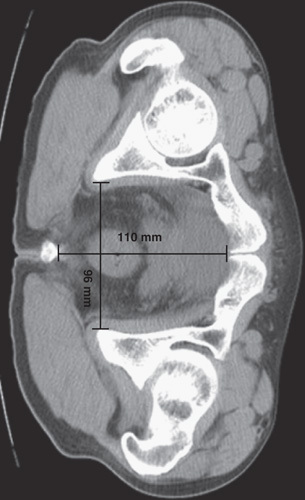
Measurements of pelvic dimensions: axial computed tomographic image showed a transverse interspinous distance of 96 mm, and anteroposterior sacrum-pubis distance of 110 mm.
Surgical Procedures
All patients underwent laparoscopic APR using well-described standard methods but with minor modifications.16 All patients’ hips were raised by 5 cm with a pad during the surgery. This was the position also used for computed tomographic imaging, with a purpose to diminish the sacral curve. The primary operative hole, with 12-mm trocar, was placed ∼3 cm below the right lower quadrant of McBurney point, to avoid injury to the inferior epigastric and external iliac vessels. The laparoscopic resection was finished until exposing the levator ani.
Statistical Analysis
Quantitative data were presented as mean±SD. Data transformation by square root was applied to the operative time and blood loss during surgery, to meet the normality requirement. Operative time and blood loss were standardized by minusing their mean and then dividing by their SD.17 Student t test or χ2 test was applied to examine the difference of each variable as indicated. Linear regression was utilized to determine the relationships between the patients’ variables and endpoints (operative time, blood loss during surgery), whereas logistic regression was applied for postoperative morbidity. After univariate analysis, variables with a P<0.25 were selected for multivariate analysis. A multivariate analysis was performed using a multiple linear regression or logistic model, with a stepwise (forward selection/backward elimination) method. The statistical analysis was performed using SAS software (SAS Institute Inc., Cary, NC), and P<0.05 was considered to be significant.
RESULTS
Patients and Tumor Characteristics
This study consisted of 117 patients with rectal adenocarcinomas (2 to 5 cm from anal verge) who underwent laparoscopic APR with total mesorectal excision (Table 1). In total, 67 (57.3%) were male and 48 (42.7%) were female. The mean age of all patients was 57.6±12.4 years. Male patients (59.0±12.0) were 3.3 years on an average older than female patients (55.7±12.9) (P=0.1624). The mean BMI was 22.4±3.1. There was no significant difference between male and female patients. Both pelvic parameters (interspinous and pubis-sacrum distances) were significantly larger in females compared with males (both P<0.0001) (Table 1).
TABLE 1.
Patients’ Demographic and Anthropometric Features
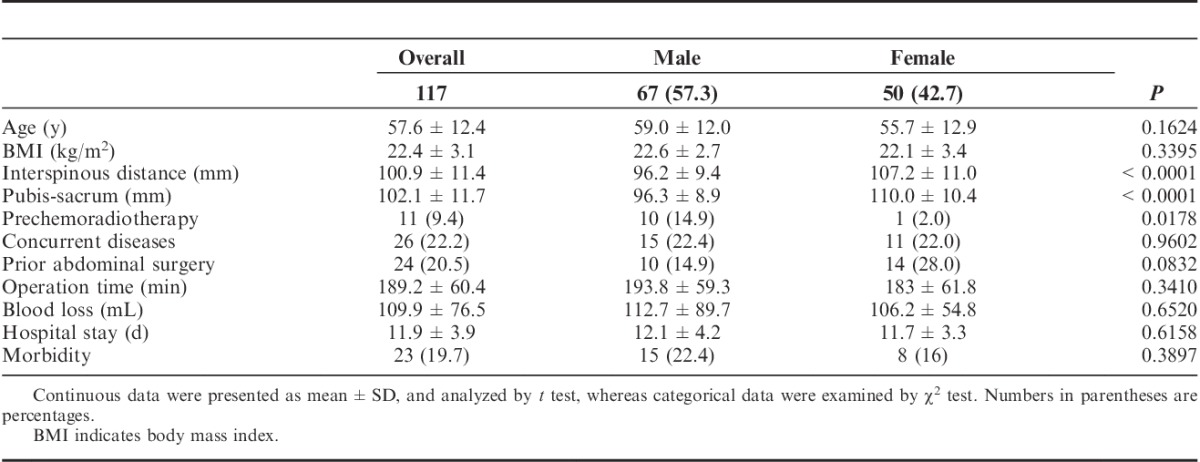
Preoperative chemotherapy and radiotherapy were administered in 11 (9.4%) patients (Table 1). Concurrent diseases occurred in 26 (22.2%) patients. A total of 24 (15.5%) patients had prior abdominal surgery. Females (29.3%) had a significantly higher rate of prior abdominal surgery than males (10.3%) (P=0.0019).
The average operative time was 189.2±60.4 minutes. Males had a longer operative time than females, by 10 minutes, but this was nonsignificant (P=0.3410) (Table 1). The average blood loss during surgery was 109.9±76.5 mL. Males and females had an average blood loss of 112.7±89.7 and 1106.2±54.8 mL, respectively (P=0.6520). Males (12.1±4.2 d) and females (11.7±3.3 d) had a similar lengths in hospital stays, following surgery (P=0.6158).
Postoperative morbidity occurred in 23 (19.7%) patients. Fifteen male patients (22.4%) and 8 female patients (16%) developed morbidity after surgery (Table 1). These morbidities included 3 case of perineal bleeding, 3 cases of incomplete intestinal obstruction, and 17 cases of infection, including perineal infection (11 cases), and other sites (6 cases). No positive longitudinal resection margins were identified. No conversion to open surgery happened and no patient died from the surgery.
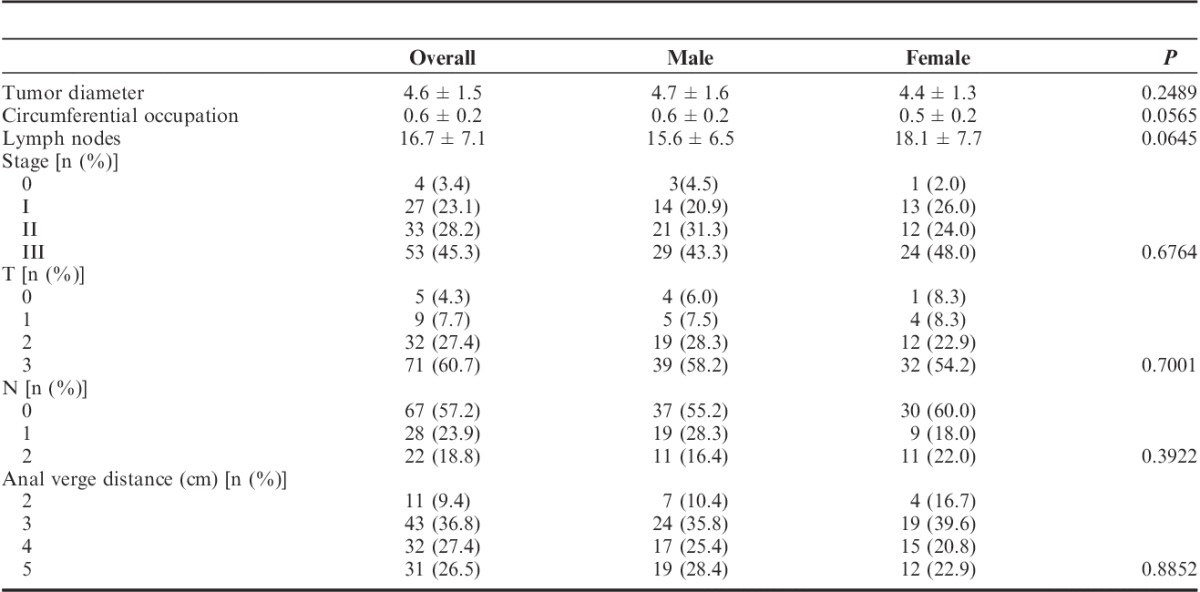
Pathologic information of tumors was summarized in Table 2. Male and female patients had similar features in tumor diameter, the degree of tumor circumferential occupation, number of lymph nodes collected, staging, TNM phases, and tumor distance to anal verge.
TABLE 2.
Anatomopathologic Features of Tumors
Factors Related to Operative Difficulty in Overall Patients
Univariate analysis showed that BMI (P=0.0005), interspinous distance (P=0.0011), tumor distance from anal verge (P=0.0123), prior abdominal surgery (P=0.0286), concurrent diseases (P=0.0026), and preoperative chemoradiotherapy (P=0.0106) were significantly associated with operative time. Multivariate analysis showed BMI (estimate=0.07, P=0.056), interspinous distance (estimate=−0.02, P=0.0011), tumor distance to anal verge (estimate=−0.17, P=0.0355), prior abdominal surgery (estimate=0.51, P=0.0180), concurrent diseases (estimate=0.49, P=0.0122), and preoperative chemoradiotherapy (estimate=0.67, P=0.0146) were predictors for operative time (Table 3).
TABLE 3.
Determinants for Operative Time
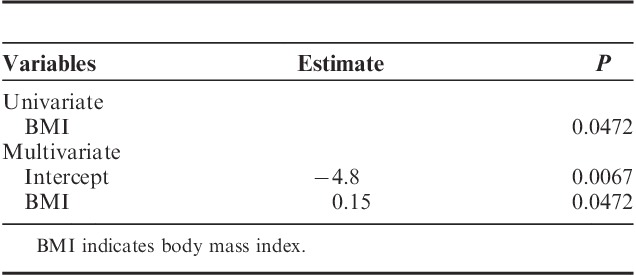
Our results show that both age and concurrent diseases were the predictors for standardized blood loss during surgery, in univariate and multivariate linear regression models (Table 4). Univariate and multivariate logistic regression analysis showed that BMI was the only factor (estimate=0.15, P=0.0472) predictable for postoperative morbidity in multivariate analysis (Table 5).
TABLE 4.
Determinants for Blood Loss
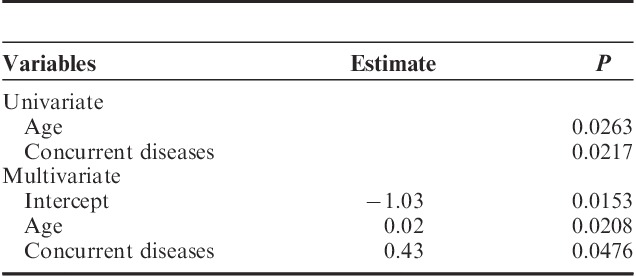
TABLE 5.
Determinants for Postoperative Morbidity
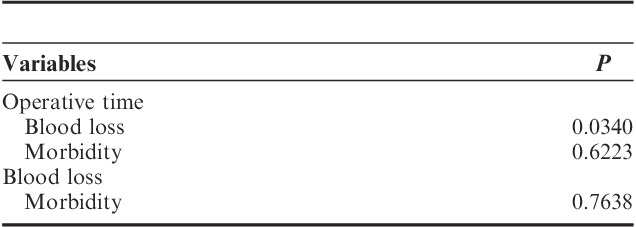
Our results showed that operative time was significantly associated with blood loss in patient’s overall (P=0.0340). In contrast, both operative time and blood loss were not predictors for postoperative morbidity (Table 6).
TABLE 6.
Interrelationship Among Different Endpoints
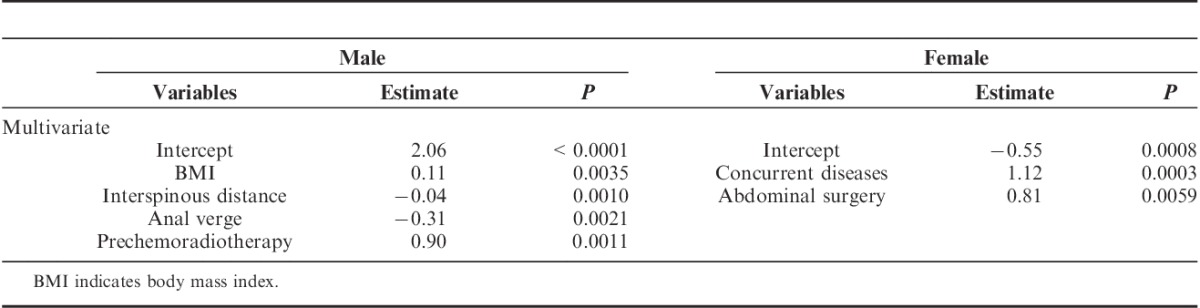
Sex has previously been considered a factor in influencing operative difficulty.15,18 Males and females have significantly different pelvic sizes, but no significant difference in operative time. This suggests that males and females have different predicting factors. Our results showed sex differences in factors impacting operative time. BMI (estimate=0.11, P=0.0035), interspinous distance (estimate=−0.04, P=0.0010), and preoperative chemoradiotherapy (estimate=0.90, P=0.0011), were significantly associated with the standardized operative time in males based on multivariate analysis (Table 7). In females, prior abdominal surgery (estimate=1.12, P=0.0003) and concurrent diseases (estimate=0.81, P=0.0059) were associated with the standardized operative time (Table 7).
TABLE 7.
Determinants for Standardized Operative Time in Males and Females
DISCUSSION
The operative time for laparoscopic resection is influenced by a surgeon’s skills10,19 and different surgical procedures. Our current study selects patients who underwent laparoscopic APR for ultra-low rectal cancer (2 to 5 cm from anal verge). All these surgeries are completed by the same expert surgeon. With this homogenous patient population, our results show that BMI, interspinous distance, tumor distance to anal verge, prior abdominal surgery, concurrent diseases, and preoperative chemoradiotherapy are the main factors that can predict operative time. We find that age and concurrent diseases are predictable for blood loss, and that BMI is a predictor for postoperative morbidity.
Great efforts have been made to identify predictors and establish practical models which can be applied in clinics, to estimate operative difficulty. The predicted operative difficulty will help surgical trainees in selecting proper cases, as well as informing patients concerning possible operative risks. A previous study successfully estimates operative difficulty using a scoring system.20 The scoring system transforms continuous data into categorical ones, which may decrease statistical sensitivity. The best way to identify reliable predictors is from data of a large sample size. Aside from accumulations of sample sizes with time, 1 alternate approach is to perform a meta-analysis. It is observed that operative time is a commonly used endpoint to estimate operative difficulty. However, numerous studies report quite different operative times,9,12,14,15,21 which will make it impossible to directly pool these data. We have introduced the standardized operative time which indirectly indicates the percentile of operative difficulty.17 This novel standardized endpoint enables to researchers to enlarge sample sizes by pooling data from published studies, and built reliable predicting models for clinical use. For this purpose, we propose an online system to store original data of all published studies.
The laparoscopic resection for rectal cancer is performed in the pelvis with a very limited working space. Many pelvic parameters have been used to determine their predicting role on operative difficulty for laparoscopic sphincter preserving resection of rectal cancer. Results are still inconsistent regarding which pelvic parameter is the best predictor. Smaller pelvic outlet,9 a less acutely curved sacrum, a narrow transverse intertuberous distance,22 shorter pubic coccyx axis,15 or transverse interspinous distance,23 and a smaller lower pelvic diameter15 are related to longer operative time. Wang et al12 report that interacetabular, anatomic transverse, interischial, intertuberous distance, distance between the coccyx and symphysis, the angles of the lower border of the symphysis pubis, upper border of symphysis pubis, and sacral promontory are inversely related to operative time. Ogiso et al10 examine 5 pelvic parameters: anteroposterior and transverse diameters in the pelvic inlet, anteroposterior and transverse diameters in the pelvic outlet, and the pelvic depth. None of them are related to operative time. Killeen et al22 report that a larger pelvic outlet is associated with longer operative time. Our study shows that interspinous distance is a predictor for operative time of laparoscopic APR. More data with larger sample size are needed to identify which pelvis parameters weigh heaviest in influencing operative difficulty.
Greater mesorectal volume in patients with higher BMI restricts the operative field and increases operative time. Majority of patients have normal BMI in our study. Our results show that BMI is a predictor for both operative time and postoperative morbidity. Our further analysis reveals that BMI is a good predictor for operative time in men, but not for women. The predicting role of BMI for laparoscopic sphincter preserving resection of rectal cancer has been observed in previous studies15; however, other researchers have shown that visceral fat may be a better predictor of operative difficulty than BMI.21 Our study suggests that BMI is an easily obtainable and useful parameter in predicting operative difficulty.
One interesting finding of the current study is the fact that all 3 preoperative clinical factors, prior abdominal surgery, concurrent diseases (hypertension and/or diabetes mellitus), and preoperative chemoradiotherapy are predictable for operative difficulty. In patients with mild or moderate hypertension, drugs used in chronic treatment may increase the need for active management of hypertensive episodes. Drosdeck et al24 reports that diabetes is a significant risk factor for incisional hernias. In contrast, no association between hypertension, diabetes, and postoperative morbidity early bowel obstruction is observed in another study.25 Prior abdominal surgery may increase tissue adhesion and fibrosis, thus increases operative time. In a study of 1000 consecutive patients, who received laparoscopic colorectal resections, prior abdominal surgery significantly increased the rate of conversion, inadvertent enterotomy, postoperative ileus, reoperation, and operative time.26 Preoperative chemoradiotherapy reduces tumor size, and improves the operative field. Previous studies have revealed the safety of rectal surgery after chemoradiotherapy.14,27–29 In contrast, preoperative chemotherapy may increase technical difficulty due to tissue edema, fibrosis, extensive mist, and exudates. All may lead to a greater number of postoperative complications in patients. Our study strongly suggests that patients’ preoperative clinical conditions should be included when estimating the operative difficulty.
Limitations of this study include its retrospective nature, as well as its moderate sample size. Because of the extra costs, only 9.4% patients are willing to undertake preoperative chemoradiotherapy. The average of our patients’ BMI is much lower than that of population in western countries, but it is comparable with Chinese colorectal cancer patients reported in a recent study.30 It is observed that there is no patient in our study having a positive longitudinal resection margin, conversion to open surgery, or surgical mortality.
CONCLUSIONS
Using this highly homogenous patient population, we have identified that age, BMI, pelvic inlet, tumor diameter, and temporary diversion influence the difficulty of performing laparoscopic APR for ultra-low rectal cancer. Standardized operative time will make it possible to significantly increase sample size by pooling data from published studies to build reliable models thus predicting operative difficulty for clinical use.
Footnotes
Supported by Zhejiang Medical Technology and Education (2011RCA010).
The authors declare no conflicts of interest.
REFERENCES
- 1.Nussbaum DP, Speicher PJ, Ganapathi AM, et al. Laparoscopic versus open low anterior resection for rectal cancer: results from the national cancer data base. J Gastrointest Surg. 2015;19:124–131. Discussion 131–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. [DOI] [PubMed] [Google Scholar]
- 3.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. [DOI] [PubMed] [Google Scholar]
- 4.Leung KL, Kwok SP, Lam SC, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187–1192. [DOI] [PubMed] [Google Scholar]
- 5.Bonjer HJ, Deijen CL, Abis GA, et al. COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332. [DOI] [PubMed] [Google Scholar]
- 6.Laurent C, Leblanc F, Wutrich P, et al. Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg. 2009;250:54–61. [DOI] [PubMed] [Google Scholar]
- 7.Zhang FW, Zhou ZY, Wang HL, et al. Laparoscopic versus open surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Asian Pac J Cancer Prev. 2014;15:9985–9996. [DOI] [PubMed] [Google Scholar]
- 8.Poylin V, Curran T, Lee E, et al. Laparoscopic colectomy decreases the time to administration of chemotherapy compared with open colectomy. Ann Surg Oncol. 2014;21:3587–3591. [DOI] [PubMed] [Google Scholar]
- 9.Akiyoshi T, Kuroyanagi H, Oya M, et al. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery. 2009;146:483–489. [DOI] [PubMed] [Google Scholar]
- 10.Ogiso S, Yamaguchi T, Hata H, et al. Evaluation of factors affecting the difficulty of laparoscopic anterior resection for rectal cancer: “narrow pelvis” is not a contraindication. Surg Endosc. 2011;25:1907–1912. [DOI] [PubMed] [Google Scholar]
- 11.Akagi T, Inomata M, Etoh T, et al. Multivariate evaluation of the technical difficulties in performing laparoscopic anterior resection for rectal cancer. Surg Laparosc Endosc Percutan Tech. 2012;22:52–57. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Xiao Y, Qiu H, et al. Factors affecting operating time in laparoscopic anterior resection of rectal cancer. World J Surg Oncol. 2014;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamabe A, Takemasa I, Uemura M, et al. Feasibility of single-port laparoscopic surgery for sigmoid colon and rectal cancers and preoperative assessment of operative difficulty. J Gastrointest Surg. 2014;18:977–985. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara S, Watanabe T, Fukushima Y, et al. Safety and factors contributing to the difficulty of laparoscopic surgery for rectal cancer treated with preoperative chemoradiotherapy. Tech Coloproctol. 2014;18:247–255. [DOI] [PubMed] [Google Scholar]
- 15.Targarona EM, Balague C, Pernas JC, et al. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg. 2008;247:642–649. [DOI] [PubMed] [Google Scholar]
- 16.Matsuhashi N, Takahashi T, Nonaka K, et al. Laparoscopic technique and safety experience with barbed suture closure for pelvic cavity after abdominoperineal resection. World J Surg Oncol. 2013;11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Li Q, Fan Y, et al. Factors predicting difficulty of laparoscopic low anterior resection for rectal cancer with total mesorectal excision and double stapling technique. PloS one. 2016;11:e0151773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg. 2004;240:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogiso S, Yamaguchi T, Hata H, et al. Introduction of laparoscopic low anterior resection for rectal cancer early during residency: a single institutional study on short-term outcomes. Surg Endosc. 2010;24:2822–2829. [DOI] [PubMed] [Google Scholar]
- 20.Veenhof AA, Engel AF, van der Peet DL, et al. Technical difficulty grade score for the laparoscopic approach of rectal cancer: a single institution pilot study. Int J Colorectal Dis. 2008;23:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seki Y, Ohue M, Sekimoto M, et al. Evaluation of the technical difficulty performing laparoscopic resection of a rectosigmoid carcinoma: visceral fat reflects technical difficulty more accurately than body mass index. Surg Endosc. 2007;21:929–934. [DOI] [PubMed] [Google Scholar]
- 22.Killeen T, Banerjee S, Vijay V, et al. Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg Endosc. 2010;24:2974–2979. [DOI] [PubMed] [Google Scholar]
- 23.Baik SH, Kim NK, Lee KY, et al. Factors influencing pathologic results after total mesorectal excision for rectal cancer: analysis of consecutive 100 cases. Ann Surg Oncol. 2008;15:721–728. [DOI] [PubMed] [Google Scholar]
- 24.Drosdeck J, Harzman A, Suzo A, et al. Multivariate analysis of risk factors for surgical site infection after laparoscopic colorectal surgery. Surg Endosc. 2013;27:4574–4580. [DOI] [PubMed] [Google Scholar]
- 25.Masoomi H, Kang CY, Chaudhry O, et al. Predictive factors of early bowel obstruction in colon and rectal surgery: data from the Nationwide Inpatient Sample, 2006-2008. J Am Coll Surg. 2012;214:831–837. [DOI] [PubMed] [Google Scholar]
- 26.Franko J, O’Connell BG, Mehall JR, et al. The influence of prior abdominal operations on conversion and complication rates in laparoscopic colorectal surgery. JSLS. 2006;10:169–175. [PMC free article] [PubMed] [Google Scholar]
- 27.Denost Q, Quintane L, Buscail E, et al. Short-and long-term impact of body mass index on laparoscopic rectal cancer surgery. Colorectal Dis. 2013;15:463–469. [DOI] [PubMed] [Google Scholar]
- 28.Valero G, Lujan JA, Hernandez Q, et al. Neoadjuvant radiation and chemotherapy in rectal cancer does not increase postoperative complications. Int J Colorectal Dis. 2003;18:495–499. [DOI] [PubMed] [Google Scholar]
- 29.Akiyoshi T, Kuroyanagi H, Oya M, et al. Safety of laparoscopic total mesorectal excision for low rectal cancer with preoperative chemoradiation therapy. J Gastrointest Surg. 2009;13:521–525. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Zheng R, Zhang M, et al. Incidence and mortality of colorectal cancer in China, 2011. Chin J Cancer Res. 2015;27:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


