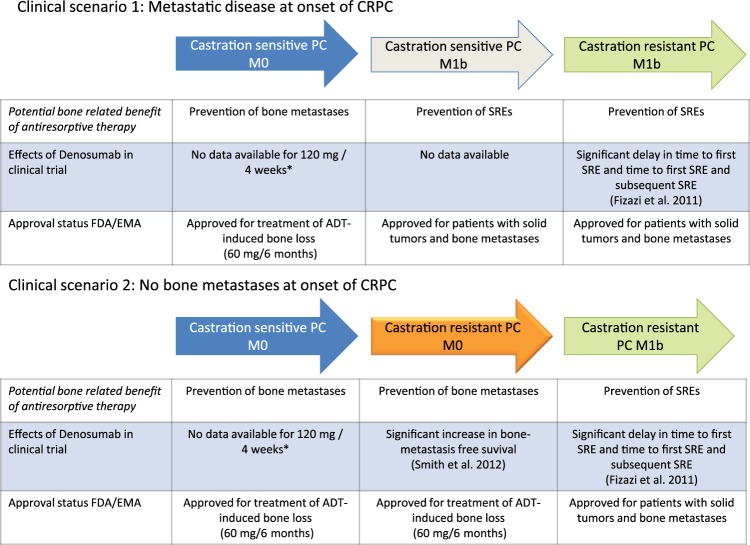
Figure 1.
Data from clinical trials and approval status of denosumab (120 mg every 4 weeks) in different disease contexts of patients with advanced prostate cancer. Green arrows indicate clinical situations with positive data from phase III clinical trials and FDA/EMA approval. The orange arrow indicates positive data from a phase III trial but without FDA/EMA approval. The grey arrow indicates approval but lack of data from a phase III trial. * In patients with ADT-induced bone loss, 60 mg denosumab every 6 months has been shown to improve bone mineral density and to reduce the risk of vertebral fractures.
ADT, androgen deprivation therapy; CRPC, castration-resistant prostate cancer; EMA, European Medicines Agency; FDA, Food and Drug Administration; PC, prostate cancer; SRE, skeletal-related event.