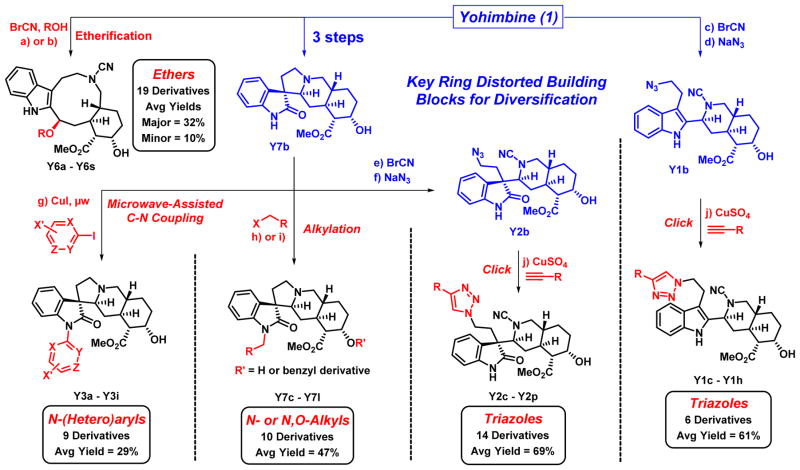
Scheme 2.
Library synthesis to afford sub-libraries of yohimbine ring-distorted compounds. Reagents and Conditions: a) BrCN (3.0 eq.), THF:H2O (2.5:1), rt, 23 h, 57%; b) BrCN (3.0 eq), alcohol:CHCl3 (1:3), rt (or reflux for Y6j-Y6o), 6.5 h (major diastereomer yields: 7 – 55%; minor diastereomer yields: 7 – 21%); c) BrCN (3.0 eq.), DMF, μw, 100 °C, 2.75 min, 56%; d) NaN3 (3.0 eq.), DMF, rt to 75 °C, 22 h, 91%; e) BrCN (3.0 eq), DMF, rt to 60 °C, 22 h, 52%; f) NaN3 (3.0 eq), DMF, rt to 80 °C, 16.5 h, 99%; g) Aryl iodide (1.1 eq.), DMEDA (0.70 eq.), CuI (0.35 eq.), MeCN, μw, 160 °C, 13 min, 20 – 37%; h) alkyl halide (1.9 eq.), K2CO3 (3.8 eq.), DMF, rt, 15 h, 44 – 80%; i) NaH (4.3 eq.), THF, 0 °C; then benzyl bromide (2.0 eq.), THF, 0 °C to rt, 16 h, 29 – 66%; j) alkyne (3.0 eq.), anhydrous CuSO4 (0.5 eq.), sodium ascorbate (1.5 eq.), tBuOH:H2O (1:2), CH2Cl2, rt, 2 h, Y1 yields: 19 – 78%, Y2 yields: 50 – 78%. BrCN = cyanogen bromide, DMEDA = N,N′-dimethylethylenediamine.