Abstract
Objective
Phenotypic heterogeneity in Tourette syndrome (TS) is partly due to complex genetic relationships between TS, obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder (ADHD). Identifying symptom-based endophenotypes across diagnoses may aid gene-finding efforts.
Method
3494 individuals recruited for genetic studies were assessed for TS, OCD, and ADHD symptoms. Symptom-level factor and latent class analyses were conducted in TS families and replicated in an independent sample. Classes were characterized by comorbidity rates and proportion of parents. Heritability and TS-, OCD-, and ADHD-associated polygenic load were estimated.
Results
We identified two cross-disorder symptom-based phenotypes across analyses: symmetry (symmetry, evening up, checking obsessions; ordering, arranging, counting, writing-rewriting compulsions, repetitive writing tics) and disinhibition (uttering syllables/words, echolalia/palilalia, coprolalia/copropraxia and obsessive urges to offend/mutilate/be destructive). Heritability estimates for both endophenotypes were high (disinhibition factor= 0.35, SE=0.03, p= 4.2 ×10−34; symmetry factor= 0.39, SE=0.03, p= 7.2 ×10−31; symmetry class=0.38, SE=0.10, p=0.001). Mothers of TS probands had high rates of symmetry (49%) but not disinhibition (5%). Polygenic risk scores derived from a TS genome-wide association study (GWAS) were associated with symmetry (p= 0.02), while risk scores derived from an OCD GWAS were not. OCD polygenic risk scores were associated with disinhibition (p =0.03), while TS and ADHD risk scores were not.
Conclusions
We identified two heritable TS-related endophenotypes that cross traditional diagnostic boundaries. The symmetry phenotype correlated with TS polygenic load, and was present in otherwise “TS-unaffected” mothers, suggesting that this phenotype may reflect additional TS (rather than OCD) genetic liability that is not captured by traditional DSM-based diagnoses.
Keywords: Tourette syndrome, attention-deficit/hyperactivity disorder, obsessive-compulsive disorder, genetics, cross-disorder, latent variable modeling
Introduction
Tourette syndrome (TS) is highly heritable(1), yet etiological and phenotypic heterogeneity, including high rates of co-occurrence with attention deficit/hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD)(2–4), have hampered gene-finding efforts(5–7). Decreasing the observed phenotypic heterogeneity, for example using latent variable modeling, may improve efforts to clarify TS pathophysiology and genetic architecture by identifying more homogeneous TS-related endophenotypes(8). In particular, the simultaneous use of distinct and complementary latent modeling approaches, such as exploratory factor analysis, to identify subsets of symptoms that group together, and latent class analysis, to identify subgroups of individuals based on patterns of symptom expression, can provide convergent evidence in support of a novel phenotypic subtype (endophenotype) that might better capture the underlying genetic liability and/or neural circuitry of complex disorders such as TS(9).
Only a few studies have previously used these multivariate methods to explore TS-associated symptom patterns(10–15). These studies identified three to five symptom groups including simple tics, complex tics, compulsive behaviors (or OCD), aggressive behaviors (or ADHD), and self-injurious behaviors (summarized in (16)). However, prior studies have been small, and have not used the full range of potentially relevant symptoms(16). In addition, few examined familial patterns of the identified factors, and no information exists about the heritability of the derived symptom subtypes or their genetic relationships to categorical TS and OCD diagnoses (16). Thus, the aims of this study were to: 1) identify and characterize unique TS-related endophenotypes with tic, OCD, and ADHD symptom data using exploratory factor and latent class analyses in a large sample of TS-affected families, 2) replicate the identified factors and latent classes in an independent sample, and 3) estimate their prevalence in parents of TS-affected probands, heritability, as well as their TS- and OCD-associated polygenic burden to determine their utility for genetic studies of TS and/or OCD.
Methods
Samples
The discovery sample included 3,850 individuals from 1,365 families collected by the Tourette Syndrome Association International Consortium for Genetics for genetic studies between 1992 and 2011. Participants were referred from TS specialty clinics in the United States, Canada, Great Britain, and the Netherlands, and from the US Tourette Association of America (formerly the Tourette Syndrome Association). Probands were defined as the first identified TS-affected individual in the family. Additional family members were subsequently recruited and assessed. Inclusion criteria for probands were: age ≥6 years old, established TS diagnosis, and availability of living parents for family-based genetic analyses. Exclusion criteria included: intellectual disability and tics caused by neurologic disorders other than TS. For children, parents and children were interviewed jointly or separately, depending on the family’s preference. For adults, parents were interviewed whenever possible to corroborate data. Families were ascertained as affected sib-pairs (two or more TS-affected siblings plus parents; N=283) or trio families (TS-affected individuals plus both parents; N=1082). The sib-pair families analyzed in our previous study are included in the current study(11). Due to the design of the original genetic study, sib-pair families were excluded at the time of enrollment if both parents had chronic tics or OCD. No such exclusions were made for trio families. Probands were excluded from analysis if they were missing all responses for any diagnostic subset of items (e.g., all responses to tic symptom items). Family members were excluded if they were missing all responses regarding tics. Additionally, cases were excluded if family role data was missing (e.g., unknown whether individual was a proband or parent) or symptom data were not verified by clinicians (ST1shows final N for each analysis). Missing data patterns did not differ by site.
A replication sample derived from the same sources under the same protocol was used for independent replication of the findings. This sample included 906 individuals from 565 families collected between 2003 and 2013.
All participants provided written informed consent (parental consent and written assent were obtained for individuals under 18). This study was approved by the Institutional Review Boards of all participating sites.
Procedure
Research staff administered clinical assessments using a standardized protocol. Demographic data, tic, OCD, and ADHD symptoms were assessed using a comprehensive, validated, semi-structured interview, the Tic and Comorbid Symptom Inventory (6, 17)(ST2). Psychiatric diagnoses were validated using a best-estimate process (SA1)(18).
Statistical Analyses
Descriptive statistical analyses were calculated using SPSS version 19. MPlus version 7.1 was used for latent variable modeling(19). PLINK was used to generate polygenic risk scores(20). Statistical analyses were conducted with R (v2.1). The R package “stats” (lm) was used to calculate R2 reported for polygenic risk score analyses. Significance for the analyses to characterize the latent classes and heritability analyses was conservatively set at P< 0.005 to account for multiple testing. Given the fact that the majority of tests were highly correlated with one another, a strict Bonferroni correction would be overly conservative. As such, we elected to set our selection threshold to p<0.005 to account for multiple testing. Because the polygenic burden analyses were exploratory in nature, they were not corrected for multiple testing.
Exploratory factor analyses
Exploratory factor analyses were conducted on symptom data using robust weighted least squares estimation, as recommended for dichotomous variables(21), and oblique rotation (geomin). Data were limited to probands to examine independent cases. The factor solution was chosen based on the following criteria: “elbow” of the scree plot, eigenvalue >1, clinical interpretability, the presence of minimal cross-loading (i.e., a single item loading on ≥1 factor at ≥0.40), and fit statistics (SA2) (22–24). Within each factor model, items were retained if factor loadings were ≥0.40; items that loaded ≥0.40 on two factors were retained on both. Items with loadings <0.40 were excluded from the final model. Items that failed to load on any factor were excluded. Cronbach’s alpha was calculated for each factor.
Latent class analyses
Models with 2–6 classes were fit in probands and replicated with probands plus family members for heritability analyses. The lowest Bayesian Information Criterion and results of the Lo, Mendel, and Rubin likelihood ratio test were used to determine the number of classes to retain(24). Specifically, the lowest Bayesian Information Criterion and a significant likelihood ratio test (p<0.05) were used to indicate good fit. If these criteria left the model choice unclear, the clinical interpretability of the solutions was examined (i.e., if clinically relevant patterns distinguished the classes in one solution but not another). Classes were labeled according to the group of symptoms that individuals in the class endorsed with a high frequency. We compared the rates of psychiatric comorbidity and rates of parents between classes using the auxiliary variable function of MPlus.
Replication sample analyses
Confirmatory factor analysis employing robust weighted least squares estimator was used to examine whether the best factor model fit the replication data among probands. Goodness of fit was evaluated using the comparative fit index, the non-normed fit index, and the root mean square error of approximation (25). To replicate the latent class results, we followed the same procedures used with the discovery sample.
Heritability estimates
Heritability estimates were calculated for factor sum scores and class membership within the discovery sample using the Sequential Oligogenic Linkage Analysis Routine (SOLAR) statistical package(26). SOLAR employs a variance component approach and calculates kinship coefficients using information from all available family members across generations. Families were only included if a proband was present. Although the majority of the families consisted of affected sibling pairs, there were 91 unaffected siblings, and 26 families had additional family members (including grandparents, uncles or aunts, and cousins). However, we note that, because most of the sample consisted of sibling pair families, we cannot with confidence separate shared genetic from shared environmental effects in these analyses. SOLAR automatically corrects for proband status to minimize potential ascertainment bias. Age, sex, and sex*age were used as covariates. To allow for generalization of the data and for the heritability analyses, mean factor sum scores were calculated for each participant by dividing the number of items the individual endorsed by the total number of items answered in the factor(27). We inverse normalized all mean sum scores because of the skewed distribution of the raw data. Because the probability distributions from the latent class analyses (i.e., probabilities that an individual will belong to each class from 0 [no probability] to 1 [100% probability]) approximated a binary distribution, we assigned each individual to his/her most likely class. Class membership was categorical and mutually exclusive.
Polygenic burden analyses
Polygenic burden analyses were conducted in the sample of probands who had both detailed phenotype data and genotype data to test for associations between multiple genes of small effect implicated in TS, OCD, or ADHD pathogenesis from genome-wide association study (GWAS) data (28–31) and phenotypes of interest identified from the latent variable modeling (SA3). The polygenic risk score was calculated as the sum of the number of risk alleles at each locus weighted by the allele effect size estimated from the GWAS of the discovery sample. The SNPs used in polygenic risk analyses were linkage disequilibrium pruned (r2<0.2) and their GWAS p values passing predetermined significance thresholds (p<0.01, 0.1, 0.2, 0.3, 0.4, and 0.5). A cross-validation approach was used in calculating the TS polygenic risk score to avoid overfitting. The factor sum scores for the phenotype of interest in the target sample were regressed on the polygenic risk scores and potential confounders (principal component factors that capture the population stratification and genotyping/imputation platforms) to assess the association between the novel phenotypes identified by the factor and/or latent class analyses and genomic variants implicated in TS, OCD, or ADHD risk (in aggregate) from genome-wide association studies (GWAS).
Results
Sample Characteristics
The final discovery sample included 1191 probands (254 from sib-pair families, 937 from trios) and 3494 total participants (1147 from sib-pair families and 2347 from trios) (Table 1).
Table 1.
Sample Characteristics
| Original Sample | Validation Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Probands only N=1191 |
Probands & family members N= 3494 | Probands only N= 527 |
Probands & family members N= 882 | |||||||||
| N | M | SD | N | M | SD | N | M | SD | N | M | SD | |
| Age | 1191 | 15.3 | 10.0 | 3494 | 30.6 | 17.2 | 522 | 21.4 | 15.6 | 864 | 30.0 | 17.5 |
| TS age onset | 1131 | 5.8 | 2.5 | 1692 | 6.1 | 2.7 | 521 | 6.2 | 2.8 | 569 | 6.3 | 2.8 |
| TS severity | 1181 | 11.4 | 2.6 | 3490 | 4.1 | 4.6 | 478 | 11.7 | 2.5 | 764 | 7.9 | 5.6 |
| OCD age onset | 442 | 7.1 | 4.0 | 712 | 8.2 | 5.3 | 249 | 7.4 | 3.8 | 299 | 8.0 | 4.4 |
| OCD severity | 694 | 4.3 | 3.4 | 2442 | 3.0 | 3.3 | 450 | 5.0 | 3.9 | 681 | 3.9 | 3.8 |
| f | % | f | % | f | % | f | % | |||||
|
| ||||||||||||
| Male | 1191 | 944 | 79.3 | 3494 | 2140 | 61.2 | 527 | 408 | 77.4 | 882 | 581 | 65.9 |
| Parental history of TS/CMVTD | 864 | 385 | 44.6 | - | - | - | - | - | - | - | - | - |
| OCD | 1135 | 570 | 50.2 | 3286 | 1125 | 34.2 | 504 | 219 | 43.5 | 847 | 336 | 39.7 |
| ADHD | 1116 | 628 | 56.3 | 3220 | 1013 | 31.5 | 494 | 181 | 36.6 | 830 | 293 | 35.3 |
| Mood Disorders | 498 | 132 | 26.5 | 1603 | 487 | 30.4 | - | - | - | - | - | - |
| Anxiety Disorders | 507 | 176 | 34.7 | 1620 | 515 | 31.8 | - | - | - | - | - | - |
| Disruptive Behavior Disorders | 390 | 121 | 31.0 | 662 | 192 | 29.0 | - | - | - | - | - | - |
M, mean; SD, standard deviation; f, frequency; ADHD, attention-deficit/hyperactive disorder; CMVTD, chronic motor or vocal tic disorder; OCD, obsessive-compulsive disorder; TS, Tourette syndrome
Note: Best estimate (mood, anxiety, and disruptive behavior disorder) diagnoses were not completed for the majority of the validation sample.
Exploratory Factor Analyses
We fit exploratory factor models with up to 10 factors using all 126 tic, OCD, and ADHD items simultaneously (SF1). The 8-factor model demonstrated the best fit (ST2, SF1), but substantial crossloading on this, as well as other higher factor models, cast doubt on the stability of this model. Since the ADHD items consistently crossloaded on all models tested, we subsequently fit factor models using the 108 tic and OCD items only. The 4-factor model demonstrated the best fit (Table 2, ST3-ST4, SF1): [factor 1] tics, [factor 2] OC symptoms, [factor 3] disinhibited symptoms, [factor 4] symmetry symptoms. The first factor includes most simple and complex tics as well as the OCD item, “needs to touch, tap, or rub things.” The disinhibited factor (factor 3) includes rude or obscene gestures and words, echolalia, palilalia, and animal/bird noises, plus hoarding and OCD items regarding urges to harm or offend. The symmetry factor (factor 4) includes tic items on repeatedly writing things and slower movements and OCD items on repeating routines, ordering, evening-up, and symmetry. The OCS factor (factor 2) contains the remainder of the OCD items. Fifteen items (7 OCD, 8 tics) failed to load on any of the factors. The internal consistency was good (Cronbach’s alpha was 0.77 to 0.92).
Table 2.
Cronbach’s Alpha and Factor Loadings for the 4-Factor Model
| Factor 1 Tics |
Factor 2 OCS |
Factor 3 Disinhibited |
Factor 4 Symmetry |
||
|---|---|---|---|---|---|
| Cronbach’s alpha | 0.88 | 0.92 | 0.77 | 0.87 | |
|
| |||||
| Factor loadings | |||||
| TS | Lift chin | 0.67 | 0.02 | −0.09 | −0.06 |
| TS | Knee-bending | 0.66 | 0.02 | 0.07 | 0.14 |
| TS | Teeth bearing | 0.63 | 0.10 | 0.07 | −0.17 |
| TS | Touch chin to shoulder | 0.60 | 0.04 | −0.08 | −0.08 |
| TS | Tensing buttocks | 0.58 | 0.02 | −0.16 | 0.18 |
| TS | Deep knee bending | 0.57 | −0.14 | 0.19 | 0.28 |
| TS | Quick eye turn | 0.56 | 0.13 | −0.07 | −0.04 |
| TS | Tensing abdomen | 0.56 | −0.03 | −0.15 | 0.12 |
| TS | Lip pouting | 0.56 | 0.13 | −0.04 | −0.11 |
| TS | Rolling eyes to one side | 0.55 | 0.02 | −0.10 | −0.01 |
| TS | Broadening nostrils | 0.55 | 0.20 | −0.22 | −0.08 |
| TS | Smiling | 0.54 | 0.07 | 0.03 | −0.04 |
| TS | Opening eyes wide | 0.52 | 0.10 | −0.10 | 0.01 |
| TS | Flexing or extending ankle | 0.52 | 0.12 | −0.13 | 0.07 |
| TS | Kicking | 0.52 | −0.01 | 0.32 | 0.03 |
| TS | Sticking tongue out | 0.51 | 0.06 | 0.24 | −0.13 |
| TS | Shrugging shoulders | 0.51 | 0.00 | 0.00 | −0.07 |
| TS | Nose twitching | 0.50 | 0.06 | −0.18 | −0.06 |
| TS | Squatting | 0.49 | −0.17 | 0.19 | 0.29 |
| TS | Biting tongue | 0.48 | 0.13 | −0.03 | 0.02 |
| TS | Touching | 0.48 | −0.14 | 0.22 | 0.35 |
| TS | Squinting | 0.48 | 0.13 | −0.15 | −0.01 |
| TS | Tapping | 0.48 | −0.07 | 0.12 | 0.26 |
| TS | Jerking shoulder | 0.47 | 0.00 | −0.05 | 0.02 |
| TS | Counting with fingers | 0.46 | 0.02 | 0.03 | 0.20 |
| TS | Bending or gyrating | 0.45 | −0.03 | 0.31 | 0.07 |
| TS | Pulling back pencil | 0.43 | −0.02 | 0.13 | 0.17 |
| TS | Throwing head back | 0.43 | 0.00 | −0.09 | 0.04 |
| TS | Skipping | 0.42 | −0.08 | 0.28 | 0.05 |
| TS | Chewing on lip | 0.42 | 0.15 | −0.04 | −0.11 |
| TS | Unusual postures | 0.42 | −0.03 | 0.28 | 0.09 |
| TS | Slower movements | 0.41 | −0.02 | 0.20 | 0.41 |
| OCD | Needs to touch, tap, or rub things | 0.41 | 0.13 | 0.14 | 0.39 |
| TS | Eye blinking | 0.41 | 0.15 | −0.27 | −0.04 |
| TS | Syllables | 0.40 | −0.16 | 0.40 | 0.05 |
| OCD | Contamination concern | 0.00 | 0.77 | −0.23 | −0.05 |
| OCD | Hoarding obsessions | −0.24 | 0.76 | 0.43 | −0.01 |
| OCD | Illness concern | 0.04 | 0.73 | −0.13 | 0.02 |
| OCD | Hoarding compulsions | −0.21 | 0.70 | 0.42 | −0.03 |
| OCD | Dirt or germ obsessions | −0.04 | 0.70 | −0.16 | 0.08 |
| OCD | Fears harming others if not careful | 0.03 | 0.66 | 0.14 | 0.07 |
| OCD | Fears responsible for something terrible | 0.03 | 0.65 | 0.05 | 0.19 |
| OCD | Thoughts may influence the outcome of some events if does certain things | −0.02 | 0.63 | −0.09 | 0.32 |
| OCD | Checks no self-harm | 0.03 | 0.62 | 0.08 | −0.08 |
| OCD | Fears harming others | 0.08 | 0.62 | 0.01 | 0.09 |
| OCD | Fears losing things | −0.02 | 0.62 | 0.10 | 0.18 |
| OCD | Contamination compulsions | 0.08 | 0.61 | −0.12 | 0.04 |
| OCD | Contamination obsessions | 0.22 | 0.61 | −0.08 | 0.03 |
| OCD | Checks nothing terrible | 0.05 | 0.60 | 0.11 | −0.02 |
| OCD | Fears will steal things | 0.06 | 0.60 | 0.22 | −0.16 |
| OCD | Religious obsessions | 0.06 | 0.60 | 0.03 | 0.06 |
| OCD | Concerned with bodily waste | 0.05 | 0.59 | −0.03 | 0.09 |
| OCD | Morality obsessions | 0.03 | 0.58 | −0.08 | 0.20 |
| OCD | Compulsions to prevent harm | 0.25 | 0.57 | −0.03 | 0.10 |
| OCD | Urges to offend | −0.05 | 0.57 | 0.56 | −0.10 |
| OCD | Checks no harm | 0.07 | 0.57 | 0.08 | 0.12 |
| OCD | Violent obsessions | 0.02 | 0.56 | 0.13 | 0.08 |
| OCD | Fears self-harm | 0.19 | 0.56 | −0.03 | 0.04 |
| OCD | Thoughts can influence the outcome of some events if does certain things | −0.02 | 0.54 | −0.10 | 0.33 |
| OCD | Mental rituals | −0.04 | 0.53 | 0.00 | 0.34 |
| OCD | Fears acting on an unwanted impulse | 0.09 | 0.53 | 0.29 | 0.12 |
| OCD | Self-harm urges | 0.11 | 0.52 | 0.35 | 0.01 |
| OCD | Superstitious fears | 0.02 | 0.50 | −0.14 | 0.35 |
| OCD | Sexual obsessions | 0.13 | 0.49 | 0.18 | 0.14 |
| OCD | Need confess | 0.00 | 0.49 | 0.12 | 0.18 |
| OCD | Animal obsessions | −0.08 | 0.48 | 0.00 | 0.12 |
| OCD | Need to explore surroundings | −0.14 | 0.46 | 0.42 | 0.03 |
| OCD | Concerned with a part of body or appearance | 0.02 | 0.45 | 0.05 | 0.20 |
| OCD | Superstitious behaviors | 0.03 | 0.42 | −0.08 | 0.34 |
| OCD | Urges to do sudden and reckless things | 0.11 | 0.40 | 0.30 | 0.13 |
| TS | Coprolalia | 0.25 | 0.05 | 0.71 | 0.00 |
| TS | Copropraxia | 0.27 | 0.06 | 0.66 | −0.08 |
| TS | Echolalia | 0.25 | −0.10 | 0.56 | 0.23 |
| OCD | Urges to be destructive | 0.02 | 0.51 | 0.55 | −0.02 |
| OCD | Urges to injure or mutilate others | −0.10 | 0.52 | 0.55 | −0.17 |
| TS | Words | 0.31 | 0.04 | 0.54 | 0.05 |
| TS | Palilalia | 0.27 | −0.02 | 0.50 | 0.25 |
| TS | Animal or bird noises | 0.26 | 0.06 | 0.41 | −0.11 |
| OCD | Symmetry obsessions | −0.07 | 0.09 | −0.04 | 0.82 |
| OCD | Needs certain things to be symmetrical | −0.02 | 0.06 | −0.01 | 0.76 |
| OCD | Ordering or arranging compulsions | −0.10 | 0.11 | 0.07 | 0.73 |
| OCD | Needs to have certain things evened-up | 0.08 | 0.06 | −0.05 | 0.73 |
| OCD | Thoughts about evening things up | 0.07 | 0.08 | −0.01 | 0.73 |
| OCD | Thoughts about lining things up | −0.01 | 0.09 | −0.01 | 0.71 |
| OCD | Obsessions about exactness | −0.03 | 0.20 | −0.05 | 0.70 |
| OCD | Re-reads or re-writes things | 0.06 | 0.20 | 0.05 | 0.59 |
| OCD | Needs to repeat routine activities | 0.03 | 0.14 | 0.11 | 0.58 |
| OCD | Counting compulsions | 0.11 | 0.14 | 0.05 | 0.54 |
| OCD | Has to do things the same way every time | −0.11 | 0.22 | 0.21 | 0.47 |
| OCD | Needs to know or remember certain things | 0.08 | 0.36 | 0.05 | 0.44 |
| OCD | Checks that did not make mistakes | 0.05 | 0.40 | −0.09 | 0.43 |
| TS | Writing the same letter or word over and over | 0.20 | 0.04 | 0.03 | 0.42 |
| OCD | Checks things | 0.11 | 0.35 | −0.11 | 0.42 |
Note: Items that loaded on 2 factors were assigned to both factors; primary loadings are in bold, secondary in bold and italics.
Latent Class Analyses
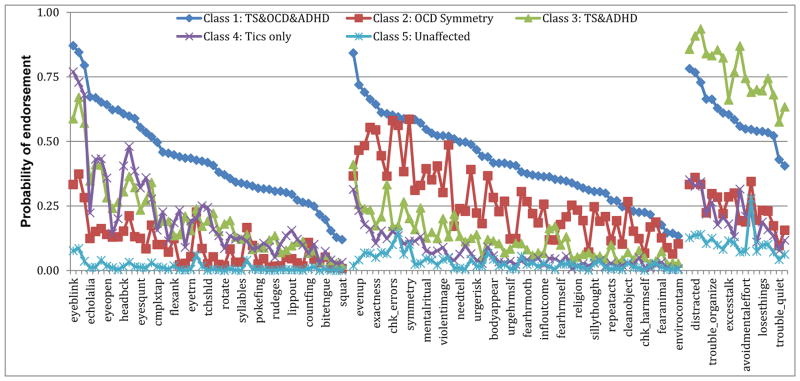
In the latent class analyses, a 4-class solution was the best fit for TS probands (ST5 & SF2). Probands in class 1 were likely to endorse symptoms from all three disorders (TS+OCD+ADHD). Probands in class 2 tended to endorse primarily OCD symmetry symptoms (about 20% of this class also endorsed a few simple tics, e.g., eye blinking). Class 3 probands endorsed high rates of tic and ADHD symptoms but minimal OCD symptoms. Finally, class 4 probands endorsed only tics. When the latent class analyses were repeated including family members, a 5-class solution was the best fit (ST5, SF2; Figure 1) and replicated the classes in the probands-only analysis, with the addition of an unaffected relative class.
Figure 1.
Probabilities of endorsing symptoms in original latent class analyses with probands & family members
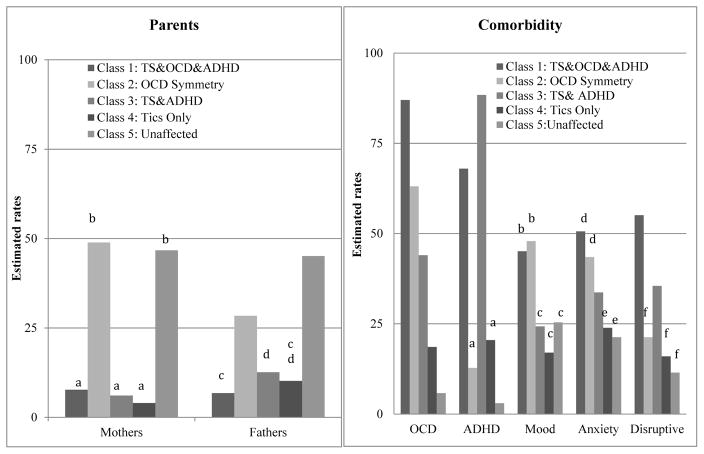
Significant differences in comorbidity rates were observed between classes (detailed in Figure 2): OCD (χ2= 1709.34, df= 4, P≤.001), ADHD (χ2= 2796.01, df= 4, P≤.001), mood (χ2= 71.17, df= 4, P≤.001), anxiety (χ2= 62.42, df= 4, P≤.001), and disruptive behavior (χ2= 60.31, df= 4, P≤.001) disorders). The proportion of mothers (χ2= 833.81, df= 4, P≤.001) and fathers (χ2= 426.12, df= 4, P≤.001) also differed between the classes; most mothers and fathers were in the unaffected (47% and 45%) and symmetry classes (49% and 29%) (Figure 2).
Figure 2.
Rates of parents (a) and comorbidity (b) for classes of probands & family members.
Note: Letters above bars indicate pairwise comparisons that are not significantly different P< 0.05. Significant differences were found for all diagnoses: OCD (χ2=1709.34, df=4, p≤.001), ADHD (χ2=2796.01, df=4, p≤.001), and mood (χ2=71.17, df=4, p≤.001), anxiety (χ2=62.42, df=4, p≤.001), and disruptive behavior (χ2=60.31, df=4, p≤.001) disorders.
Replication Analyses
Characteristics of the 882 participants (527 probands and 355 family members) included in the replication analyses were similar to those of the discovery sample (Table 1). The confirmatory factor analysis of the 4-factor model with probands demonstrated a good fit (root mean square error of approximation = 0.02; comparative fit index = 0.93, non-normed fit index = 0.93). The results of the latent class analyses in the replication sample paralleled (confirmed) the original findings in the discovery sample using probands and parents: a 5-class solution was the best fit and identified a distinct “symmetry” class in addition to TS+OCD+ADHD, TS+ADHD, tics only and unaffected classes (ST5 and SF2-SF3).
Heritability Analyses
Heritability estimates were calculated for the 4-factor and the 5-class latent class solutions (excluding the “unaffected” classes; Table 3). Heritabilities for the factors ranged from 0.25 to 0.38 (all P-values≤2.6 ×10−13). Heritabilities for the classes ranged from 0.28 (class 4, TS-only, P= .001) to 0.63 (class 5, TS+OCD+ADHD, P= 2.0×10−13).
Table 3.
Heritability estimates for classes of probands & family members.
| Latent Class Analyses | H2r | SE | p-value |
|---|---|---|---|
| Class 1: TS&OCD&ADHD | 0.63 | 0.09 | 2.0×10−13 |
| Class 2: Symmetry symptoms | 0.38 | 0.10 | 0.001 |
| Class 3: TS & ADHD | 0.47 | 0.08 | 3.3×10−29 |
| Class 4 TS only | 0.28 | 0.08 | 0.001 |
| Class 5: Unaffected | - | - | - |
| Exploratory Factor Analyses | |||
| Factor 1–Tics | 0.25 | 0.04 | 2.6 ×10−13 |
| Factor 2–OCS | 0.46 | 0.34 | 8.6 ×10−41 |
| Factor 3–Disinhibited | 0.35 | 0.03 | 4.2 ×10−34 |
| Factor 4–Symmetry | 0.39 | 0.03 | 7.2 ×10−31 |
Note: All analyses included sex, age, and sex*age as covariates. H2r is the heritability estimate.
As we identified two non-traditional patterns of symptom endorsement that might be useful for future studies of TS pathophysiology, symmetry in both the factor analyses and the latent class analyses, and disinhibited symptoms in the factor analyses, we conducted additional post-hoc heritability and polygenic burden analyses. For symmetry, we combined the classes endorsing these symptoms at high rates (i.e., class 1 and class 2). 997 individuals (29%) endorsed symmetry symptoms at high rates; the heritability for symmetry was 0.53 (SE= 0.09, P= 6.3×10−10). Only the TS+OCD+ADHD endophenotype had a higher heritability estimate (h2r= 0.63, Table 3). As noted above, the heritability for disinhibited symptoms, which was seen in the factor analyses but not the latent class analyses, was 0.35 (Table 3).
Polygenic Burden
We examined the association between TS, OCD, and ADHD polygenic burden and the two novel phenotypes, symmetry and disinhibition, in 947 TS probands with available genotype data (no genotype data are yet available on relatives). Both phenotypes were defined as continuous factor sum scores from the exploratory factor analysis. For exploratory purposes, the disinhibition phenotype was defined in two ways; 1) including the presence of hoarding symptoms in the factor sum score, and 2) excluding hoarding symptoms, as these symptoms consistently factored out from the other disinhibition symptoms in higher factor solutions. The symmetry phenotype was positively associated with TS polygenic risk score (R2 = 0.57%; P=0.02) but not with OCD or ADHD polygenic risk scores (R2 = 0.19% (negative correlation), p = 0.18 and R2 = 0.13% (negative correlation), p = 0.26, respectively) (ST6 and SF4). In contrast, the disinhibition phenotype was significantly associated with OCD polygenic risk score ((R2 = 0.52%; P=0.026) but was not significantly associated with TS or ADHD polygenic risk scores (R2 = 0.18%, p = 0.19 and R2 = 0.23%, p = 0.14, respectively). The disinhibition phenotype excluding hoarding symptoms was also significantly associated with OCD polygenic risk score (R2 = 0.56%, p = 0.021), and had a positive but not statistically significant correlation with TS and ADHD polygenic risk scores (R2 = 0.27%, p = 0.11 and R2 = 0.30%, p = 0.10, respectively)(ST6 and SF4).
Discussion
This is the first study to use multiple modeling approaches on symptom-level data in a large sample of TS-affected individuals and family members to identify heritable TS-associated endophenotypes. The use of factor and latent class analyses in the same dataset provides an opportunity to thoroughly examine these complex phenotypes; the complementary findings across the approaches are notable. These analyses extend previous work and highlight two TS-related endophenotypes (disinhibition and symmetry symptoms) of potential use in future research. The heritability and polygenic burden analyses, which are also complementary, provide insight into the possible biological underpinnings of these cross-disorder phenotypes, and their likely utility in future genetic studies aimed at identifying TS-related susceptibility variants.
The symmetry phenotype was seen in both the factor and latent class analyses, and parallels and refines the chronic tics + OCD class identified in our previous work using categorical diagnoses(11). We note that OCD + tics is now a specifier for OCD in the DSM-5, acknowledging the growing awareness that the co-occurrence of tic symptoms represents a specific subtype of this disorder(32). The identification of this endophenotype is also consistent with previous literature suggesting that individuals with TS are more likely to have symmetry symptoms, which are associated with specific neural correlates in motor and limbic circuits(33), rather than other OCD symptoms. Of note, symmetry scores were positively associated with TS aggregated polygenic risk scores but not with OCD polygenic risk score, even though these symmetry symptoms are derived from the Yale-Brown Obsessive Compulsive Scale and traditionally considered to be OC-related. This finding is also consistent with previous GWAS analyses demonstrating that OCD with and without tics have some degree of non-overlapping genetic risk(30), as well as with the clinical observations that symmetry symptoms seem to be driven by the need for things to feel “just right” (similar to premonitory urges for tics) rather than classic anxiety symptoms(34).
Additionally, in the latent class analysis, the symmetry class stood in contrast to all other classes, where categorical diagnoses appeared to be a bigger driver than symptom-level variation. Of note, the higher rate of mothers (including otherwise “unaffected” mothers of TS probands) in the symmetry class compared to the other affected classes (Figure 2) fits with prior observations that females in TS families are more likely to have OCD than tics, and suggests that symmetry may represent an alternative TS-susceptibility phenotype that may be influenced by sex(35). The high heritability of this phenotype, and the increased TS, but not OCD, polygenic risk burden in symmetry-positive individuals, provides further support for this symptom-based cross-disorder phenotype as an appropriate substrate for further genetic analyses aimed at identifying TS-associated genetic variation.
The disinhibition phenotype was identified only in the factor models; however, it was identified in a “tic-only” item level latent class analyses in the same sample (36). Interestingly, the disinhibition factor is similar to an aggressive tic factor found in a previous TS study, as well as an aggressive symptom cluster identified previously in OCD-affected individuals, combined with religious and sexual symptoms to form a “taboo” factor (12, 27). The heritability of this phenotype was among the highest identified, and although the polygenic risk score patterns were less clear for the disinhibition phenotype than for symmetry, there was some evidence for association between TS, OCD, and ADHD polygenic risk scores and the disinhibition phenotype, particularly when excluding hoarding symptoms. Although not meeting criteria for statistical significance, and clearly requiring replication in larger samples, this pattern of association between disinhibition and increased polygenic burden for TS, OCD, and ADHD suggests that this endophenotype, rather than being specific for TS, may reflect deficits in top-down cognitive control that is seen across all three of these disorders (37–40).
Additional studies, including larger scale GWAS studies, are needed to confirm and further parse the genetic and neurobiological relationships of the disinhibition phenotype to TS, OCD, and ADHD. This phenotype may also be of particular interest for neuroimaging studies of TS and OCD, again potentially correlating with impairment in top down cortical control/response inhibition, and associated patterns of dysfunctional cortico-striatal-thalamo-cortical circuitry in these disorders.(37–39)
As noted, both the symmetry and disinhibition phenotypes are relevant for future genetic studies, as both had high heritabilities and capture specific elements of the TS phenotype that might have different risk genes and/or pathophysiology than TS (or OCD) as defined using standard diagnostic criteria. Future research might also explore whether individuals endorsing these symptoms differ clinically (e.g., in terms of tic persistence or prognosis), pathophysiologically (e.g., severity of frontostriatal circuit disruption), or in treatment response.
Limitations
The primary limitation in these analyses reflects the fact that data were collected over an extended time period at many different sites. This limitation may also represent an advantage: the sample’s heterogeneity is likely to increase the generalizability of the findings. Also, statistical power was limited for some analyses, particularly for the polygenic burden analyses. Additionally, the small number of unaffected family members other than parents may limit the interpretation of the heritability estimates, as we cannot confidently separate shared genetic from shared environmental effects in the current sample. Nevertheless, the consistency of our findings across analytic approaches is striking, and provides a novel and likely fruitful avenue of investigation for future genetic and neurobiological studies of TS and its co-occurring disorders.
Supplementary Material
Acknowledgments
We wish to thank the families and patients who participated in this research and the study coordinators at each site for their assistance.
Financial support This work was supported by the National Institutes of Health, grant numbers R01MH096767 (PI: Carol Mathews), U01NS040024 (PI: David Pauls/Jeremiah Scharf), K23MH085057 (PI: Jeremiah Scharf), K02MH00508 (PI: David Pauls), and R01NS016648 (PI: David Pauls), and from the Tourette Syndrome Association.
Additional TSAICG members: Danielle Posthuma (VU University, Amsterdam); Harvey Singer (Johns Hopkins University, Baltimore); Benjamin Neale (Massachusetts General Hospital, Boston); Nancy Cox (University of Chicago, Chicago); Nelson Freimer, Giovanni Coppola (University of California, Los Angeles); Guy Rouleau (Montreal Neurological Institute, Montreal); Cathy Barr (Toronto Western Hospital, University of Toronto).
Footnotes
Conflict of interest
Drs. Darrow, Hirschtritt, Illmann, Greenberg, McGrath, and Delucchi, and Ms. Osiecki reported no biomedical financial interests or potential conflicts of interest. Drs. Grados, Sandor, McMahon, Pauls, Dion, King, Budman, Freimer, Cox, Cath, Lyon and Lee received research support from the Tourette Association of America (TAA). Dr. Cath has received speakers’ honoraria from Pfizer BV. Dr. Budman reported funding for clinical research studies from Psyadon Pharmaceuticals and Otsuka Pharmaceuticals. Dr. Sandor reported Unrestricted Educational Grants from Purdue and Shire, a speaker fee from Purdue and support for clinical research from Otsuka Pharmaceuticals. Dr. Sandor was a member of the data safety monitoring committee for Psyadon Pharmaceuticals. Dr. Scharf has received consulting fees from Nuvelation Pharma, Inc. Drs. Scharf and Mathews have received research support, honoraria and travel support from the TAA and are members of the TAA Scientific Advisory Board. None of the funding agencies for this project (NINDS, NIMH, the Tourette Syndrome Association) had any influence or played any role in a) the design or conduct of the study; b) management, analysis or interpretation of the data; c) preparation, review or approval of the manuscript. The views expressed in this publication are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
References
- 1.Pauls DL, Fernandez TV, Mathews CA, State MW, Scharf JM. The Inheritance of Tourette Disorder: A review. Journal of obsessive-compulsive and related disorders. 2014;3(4):380–5. doi: 10.1016/j.jocrd.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffey BJ, Biederman J, Smoller JW, Geller DA, Sarin P, Schwartz S, et al. Anxiety disorders and tic severity in juveniles with Tourette’s disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(5):562–8. doi: 10.1097/00004583-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Gorman DA, Thompson N, Plessen KJ, Robertson MM, Leckman JF, Peterson BS. Psychosocial outcome and psychiatric comorbidity in older adolescents with Tourette syndrome: controlled study. British Journal of Psychiatry. 2010;197(1):36–44. doi: 10.1192/bjp.bp.109.071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in tourette syndrome. JAMA Psychiatry. 2015;72(4):325–33. doi: 10.1001/jamapsychiatry.2014.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O’Roak BJ, Mason CE, et al. L-histidine decarboxylase and Tourette’s syndrome. The New England journal of medicine. 2010;362(20):1901–8. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tourette Syndrome Association International Consortium for G. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. American Journal of Human Genetics. 2007;80(2):265–72. doi: 10.1086/511052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science (New York, NY) 2005;310(5746):317–20. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 8.Worbe Y, Mallet L, Golmard J-L, Béhar C, Durif F, Jalenques I, et al. Repetitive behaviours in patients with Gilles de la Tourette syndrome: Tics, compulsions, or both? PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruderfer DM, Fanous AH, Ripke S, McQuillin A, Amdur RL, Gejman PV, et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2014;19(9):1017–24. doi: 10.1038/mp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathews CA, Jang KL, Herrera LD, Lowe TL, Budman CL, Erenberg G, et al. Tic symptom profiles in subjects with Tourette Syndrome from two genetically isolated populations. Biological Psychiatry. 2007;61(3):292–300. doi: 10.1016/j.biopsych.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Grados MA, Mathews CA. Latent Class Analysis of Gilles de la Tourette Syndrome Using Comorbidities: Clinical and Genetic Implications. Biological Psychiatry. 2008;64(3):219–25. doi: 10.1016/j.biopsych.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson MM, Althoff RR, Hafez A, Pauls DL. Principal components analysis of a large cohort with Tourette syndrome. The British journal of psychiatry : the journal of mental science. 2008;193(1):31–6. doi: 10.1192/bjp.bp.107.039909. [DOI] [PubMed] [Google Scholar]
- 13.Shytle RD, Silver AA, Sheehan KH, Wilkinson BJ, Newman M, Sanberg PR, et al. The Tourette’s Disorder Scale (TODS): Development, reliability, and validity. Assessment. 2003;10(3):273–87. doi: 10.1177/1073191103255497. [DOI] [PubMed] [Google Scholar]
- 14.Storch EA, Murphy TK, Fernandez M, Krishnan M, Geffken GR, Kellgren AR, et al. Factor-analytic study of the Yale Global Tic Severity Scale. Psychiatry Research. 2007;149(1–3):231–7. doi: 10.1016/j.psychres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Robertson MM, Cavanna AE. The Gilles de la Tourette syndrome: a principal component factor analytic study of a large pedigree. Psychiatric genetics. 2007;17(3):143–52. doi: 10.1097/YPG.0b013e328015b937. [DOI] [PubMed] [Google Scholar]
- 16.Grados MA, Mathews CA. Clinical phenomenology and phenotype variability in Tourette syndrome. Journal of psychosomatic research. 2009;67(6):491–6. doi: 10.1016/j.jpsychores.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Tourette Syndrome Association International Consortium for Genetics. A complete genome screen in sib pairs affected by Gilles de la Tourette syndrome. American Journal of Human Genetics. 1999;65(5):1428–36. doi: 10.1086/302613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Archives of General Psychiatry. 1982;39(8):879–83. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 19.Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998. [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muthén B, du Toit SHC, Spisic D. Unpublished technical report. 1997. Robust inference using weighted least squares and quadratic estimating equations in latent variable modeling with categorical and continuous outcomes. Contract No.: Report. [Google Scholar]
- 22.Preacher KJ, Zhang G, Kim C, Mels G. Choosing the optimal number of factors in exploratory factor analysis: A model selection perspective. Multivariate Behavioral Research. 2013;48(1):28–56. doi: 10.1080/00273171.2012.710386. [DOI] [PubMed] [Google Scholar]
- 23.Floyd FJ, Widaman KF. Factor analysis in the development and refinement of clinical assessment instruments. Psychological Assessment. 1995;7(3):286–99. [Google Scholar]
- 24.Loehlin JC. Goodness of Fit Indices: Latent Variable Models. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. [Google Scholar]
- 25.Brown TA. Confirmatory Factor Analysis for Applied Research. 2. New York: Guilford Press; 2015. [Google Scholar]
- 26.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. American Journal of Human Genetics. 1998;62(5):1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katerberg H, Delucchi KL, Stewart SE, Lochner C, Denys DAJP, Stack DE, et al. Symptom dimensions in OCD: Item-level factor analysis and heritability estimates. Behavior Genetics. 2010;40(4):505–17. doi: 10.1007/s10519-010-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, Derks EM, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS genetics. 2013;9(10):e1003864. doi: 10.1371/journal.pgen.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette’s syndrome. Mol Psychiatry. 2013;18(6):721–8. doi: 10.1038/mp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu D, Mathews CA, Scharf JM, Neale BM, Davis LK, Gamazon ER, et al. Cross-Disorder Genome-Wide Analyses Suggest a Complex Genetic Relationship Between Tourette’s Syndrome and OCD. Am J Psychiatry. 2015;172(1):82–93. doi: 10.1176/appi.ajp.2014.13101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, et al. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884–97. doi: 10.1016/j.jaac.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leckman JF, Denys D, Simpson HB, Mataix-Cols D, Hollander E, Saxena S, et al. Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress Anxiety. 2010;27(6):507–27. doi: 10.1002/da.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries FE, van den Heuvel OA, Cath DC, Groenewegen HJ, van Balkom AJ, Boellaard R, et al. Limbic and motor circuits involved in symmetry behavior in Tourette’s syndrome. CNS spectrums. 2013;18(1):34–42. doi: 10.1017/S1092852912000703. [DOI] [PubMed] [Google Scholar]
- 34.Fibbe LA, Cath DC, van Balkom AJ. Obsessive compulsive disorder with tics: a new subtype? Tijdschrift voor psychiatrie. 2011;53(5):275–85. [PubMed] [Google Scholar]
- 35.Como PG, LaMarsh J, O’Brien KA. Obsessive-compulsive disorder in Tourette’s syndrome. Adv Neurol. 2005;96:249–61. [PubMed] [Google Scholar]
- 36.Hirschtritt ME, Darrow SM, Illmann C, Osiecki L, Grados M, Sandor P, et al. Social disinhibition is a heritable subphenotype of tics in Tourette syndrome. Neurology. 2016 doi: 10.1212/WNL.0000000000002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, et al. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 2012;169(10):1100–8. doi: 10.1176/appi.ajp.2012.12010073. [DOI] [PubMed] [Google Scholar]
- 39.Rae CL, Hughes LE, Anderson MC, Rowe JB. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(2):786–94. doi: 10.1523/JNEUROSCI.3093-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shephard E, Jackson GM, Groom MJ. The effects of co-occurring ADHD symptoms on electrophysiological correlates of cognitive control in young people with Tourette syndrome. Journal of neuropsychology. 2015 doi: 10.1111/jnp.12071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.