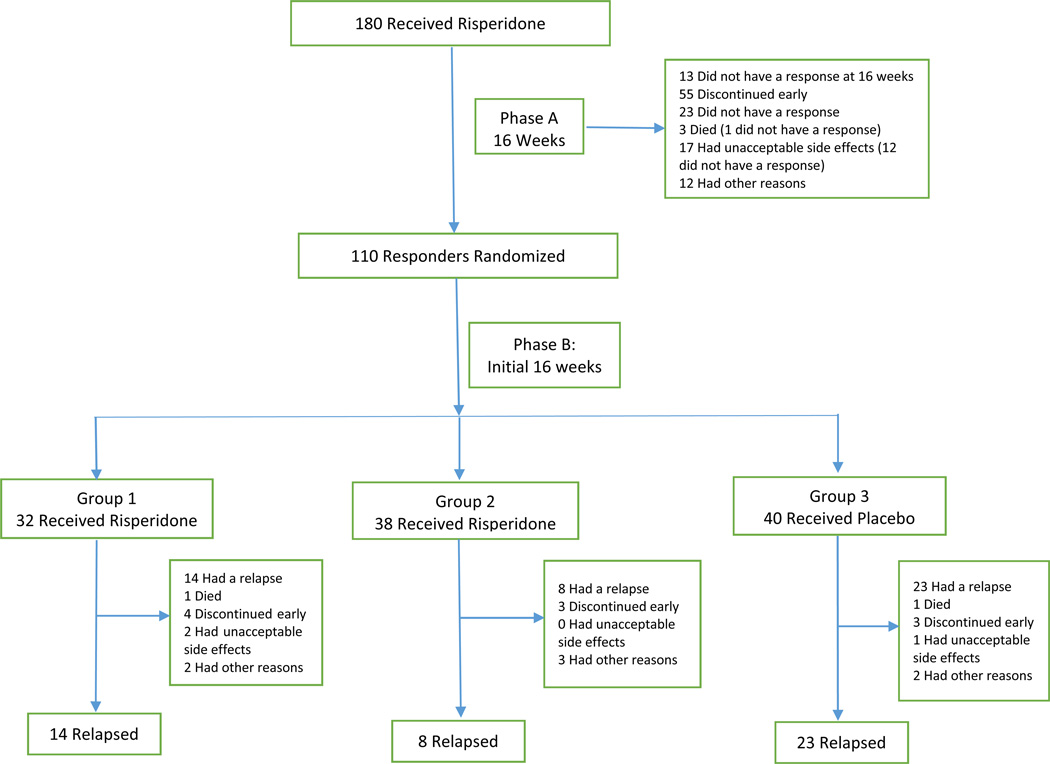
Figure 1. Patient flow through Phase A 16 weeks of open treatment and post-randomization initial 16 weeks of Phase B.
In Phase B (combining all groups), in addition to patient relapse, subjects exited the study because of death (n=2), early discontinuation (n=10), unacceptable side effects (n=3), or other reasons n=7). The final 16 weeks in the trial were not analyzed due to small sample size; full consort diagram has been previously published [6].