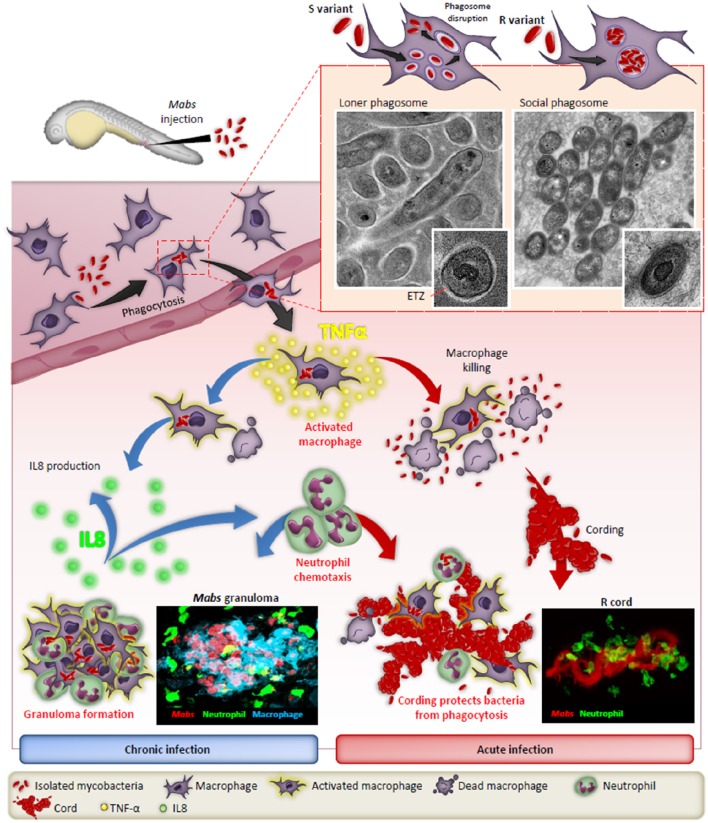
Figure 1.
The distinct fates of the smooth (S) and rough (R) variants of M. abscessus in macrophages and zebrafish. After injection in the blood flow, Mabs are rapidly phagocytozed by macrophages, either individually (S variant) in loner phagosomes or as bacterial clusters (R variant) in social phagosomes. The presence of GPL production in the S strain leads to a typical electron translucent zone (ETZ) that fills the entire space between the phagosome membrane and the mycobacterial cell wall. Loss of GPL in the R variant results in disappearance of the ETZ. In some instances, disruption of the phagosome membrane releases the S variant in the cytoplasm. Infected macrophages migrate from the vasculature the nervous tissues and become heavily infected which leads to apoptosis with the release of the S variant that are phagocytosed by newly recruited macrophages which, together with neutrophils, form protective granulomas, resulting in chronic infection. In contrast, the release of Mabs R is correlated with the emergence of extracellular serpentine cords, preventing phagocytosis of the bacilli by macrophages and neutrophils, leading to abscess formation with tissue destruction and acute infection. TNF-α plays a central role in the immunity to Mabs by activating the macrophage bactericidal response and, through IL-8 production, in neutrophil chemotaxis to the site of infection or to form protective granulomas. Adapted from Bernut et al. (2016).