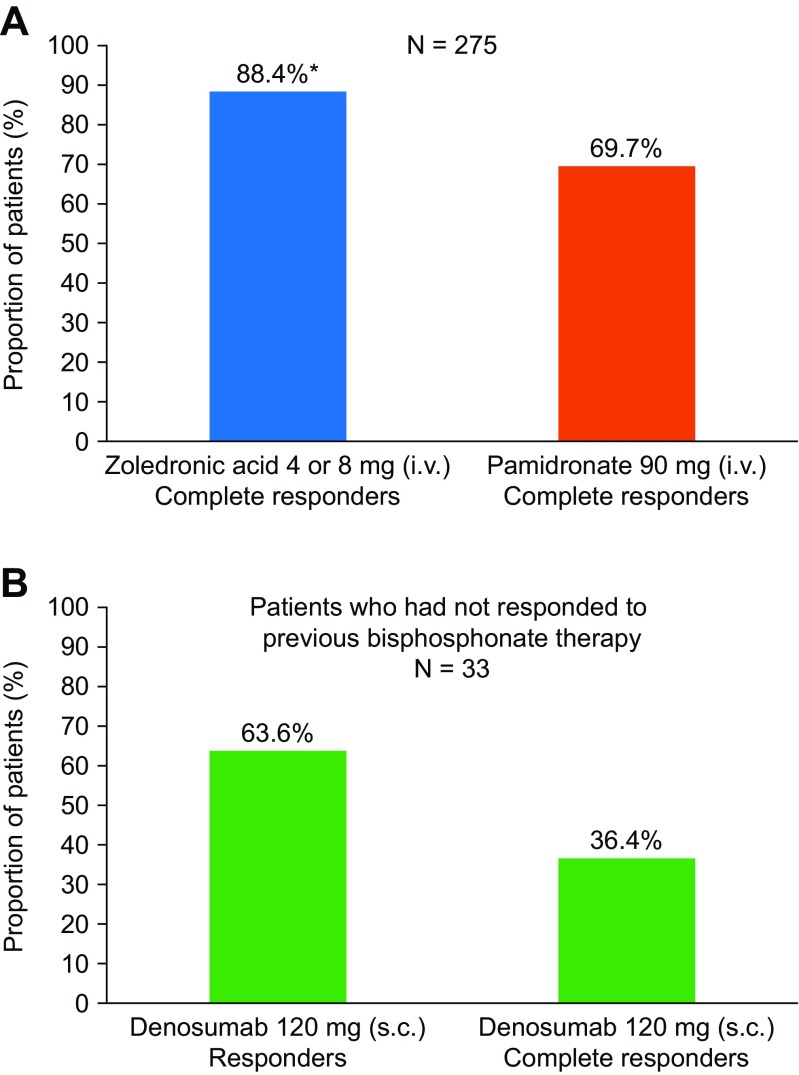
Fig. 2.
Responses to treatment with bisphosphonates or denosumab. a Proportion of patients demonstrating a complete response at day 10 in a pooled analysis of two randomised, double-blind phase 3 trials of patients with moderate-to-severe hypercalcaemia of malignancy who received zoledronic acid or pamidronate (N = 275). Complete response was defined as CSC ≤ 10.8 mg/dL (2.7 mmol/L). [52]. b Proportion of patients demonstrating a response or complete response at day 10 in a single-arm, open-label study of patients who had hypercalcaemia of malignancy (CSC levels >12.5 mg/dL [3.125 mmol/L]) despite receiving bisphosphonate treatment. During the study, patients (N = 33) received denosumab 120 mg s.c. and response was defined as CSC < 11.5 mg/dL (2.9 mmol/L; CTCAE grade 0 or 1). Complete response was defined as CSC ≤ 10.8 mg/dL (2.7 mmol/L). [53]. *P = 0.002 versus pamidronate. CSC albumin-corrected serum calcium, CTCAE Common Terminology Criteria for Adverse Events, i.v. intravenous, s.c. subcutaneous