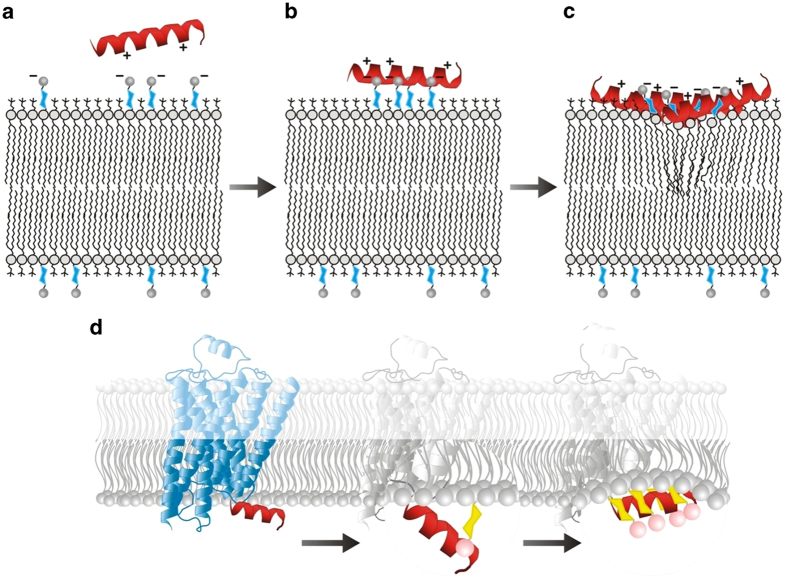
Figure 5.
Schematic for the proposed mechanism for the binding of Helix 8 to a lipid bilayer containing PIPs, as observed by DPI. Before binding (A) the positive residues of the Helix 8 peptide are attracted to the negatively charged phosphate groups on the PIPs. Upon binding (B) the helix is held above the membrane by ionic bonding between Helix 8 and PIP phospholipids. As a large amount of Helix 8 peptide binds to the membrane (C) the ratio of PIPs to peptide drops and the peptide moves into the membrane, causing disruption of its structure. (D) Schematic for the proposed mechanism by which interaction between Helix 8 and PIPs may trigger a conformational change within the context of the AT1R. The first change involves ionic binding to the phosphate groups of PIP molecules protruding from the membrane surface, pulling the helix away from the membrane. This results in the unfavourable exposure of hydrophobic residues to the hydrophilic cytosol. In the second change, the helix responds to this by returning to the inner leaflet of the membrane, lying between the phosphate groups of the PIPs and the headgroups of the more abundant lipid species.