Abstract
CuInS2 (CIS) quantum dots (QDs) have tunable photoluminescence (PL) behaviors in the visible and near infrared spectral range with markedly lower toxicity than the cadmium-based counterparts, making them very promising applications in light emitting and solar harvesting. However, there still remain material- and fabrication- related obstacles in realizing the high-performance CIS-based QDs with well-resolved Mn2+ d-d emission, long emission lifetimes as well as high efficiencies. Here, we demonstrate the growth of high-quality Mn2+-doped CuInS-ZnS (CIS-ZnS) QDs based on a multi-step hot-injection strategy. The resultant QDs exhibit a well-resolved Mn2+ d-d emission with a high PL quantum yield (QY) up to 66% and an extremely long excited state lifetime up to ~3.78 ms, which is nearly two times longer than the longest one of “green” QDs ever reported. It is promising that the synthesized Mn2+-doped CIS-ZnS QDs might open new doors for their practical applications in bioimaging and opto/electronic devices.
Manganese ions-doped (Mn2+-doped) semiconductor nanocrystals (NCs), as a lucrative alternative to semiconductor QDs, can not only maintain nearly all the intrinsic advantages of QDs, but also possess the additional merits such as larger Stokes shifts, enhanced thermal and chemical stabilities, as well as longer excited state lifetimes, which suggest their exciting and interesting applications in biomedical diagnosis, QD solar cells (QDSCs), light-emitting diodes (LEDs) and so forth1,2,3,4. Up to date, considerable interest has been attracted in the Mn2+-doped semiconductor NCs5,6,7,8,9,10,11,12,13,14. Even highly efficient Mn2+ emissions have often been achieved in the binary intrinsic/alloyed group II−VI semiconductor NCs, these hosts are limited in either needing high energy for excitation11,12 or containing the toxic elements of Cd and/or Se against the environment-friendly applications9,11,13.
Colloidal group I-III-VI NCs could offer a facility to tailor the bandgaps without the use of toxic elements, which might advance the exploration of high-performance “green” QDs15,16. Among the families of group I-III-VI semiconductors, CuInS2 (CIS) has an intrinsic direct bandgap of 1.5 eV with size-dependent luminescence behaviors in the visible and near infrared range. Meanwhile, their bandgaps can be tailored widely by growing a ZnS shell or making them be alloyed CuZnInS (CZIS) NCs with Zn, to meet the prerequisite for the well-resolved Mn2+ d-d emission, which can be expected to be the ideal “green” materials for widespread technological applications where the Mn2+ emission required17,18,19. As compared to the CZIS alloyed QDs, the CIS-ZnS core-shell counterparts with the designed growth of ZnS shells, show the remarkable advantages to offer a significantly effective surface passivation for higher PL QYs19,20,21,22,23,24, as well a more facility to control the Mn2+ positions25. For examples, Yang et al. reported the synthesis of Mn2+-doped CdS/ZnS core-shell NCs with Mn2+ at controlled radial positions used a three-step synthesis route25. Liu et al. reported the Mn:CIS/ZnS core-shell QDs, which exhibited tunable PL peaked from 542 to 648 nm20, and Ding et al. also reported the CIS/Mn:ZnS QDs had high PL QY due to the effective surface passivation by ZnS shell26. It should point out that the defect state emission is predominantly in their PL spectra and no pure atomic-like Mn2+ d-d emission was observed. Besides the improved high PL QY by the effective surface passivation, the lifetime of the PL has been considered as another important role on the performance of the QD nanodevices, since the long lifetime benefits to modifying the charge separation and recombination dynamics22,27. Luo et al. achieved a high power conversion efficiency (PCE) of 5.38% in the QDSCs with Mn:CIS/CdS core–shell QDs, showing a 14.7% higher PEC than CIS/CdS counterparts22. With respect to state-of-the-art CIS based QDs, regardless of the fact that even the Mn2+ dopants did have been incorporated, there still remain material- and fabrication- related obstacles in realizing high-performance CIS-based QDs with well-resolved Mn2+ d-d emission and high efficiency as well as long lifetimes15,28, which are important for their applications, e.g., in bioimaging, opto/electronic devices.
Here, we report the synthesis of Mn2+ doped CIS-ZnS QDs based on a multi-step hot-injection method. We mainly focus on three important topics for conferring the CIS-ZnS QDs with high optical performances: i) to grow high-quality Mn2+-doped CIS-ZnS QDs; ii) to obtain the well-resolved Mn2+ emission in CIS-ZnS QDs; iii) to make the long excited state lifetime of Mn2+ emission in CIS-ZnS QDs as well as high efficient PL QYs. The resultant CIS-ZnS QDs exhibit highly efficient and well-resolved Mn2+ d-d emission with a PL QY up to 66% and a surprisingly long lifetime up to ~3.78 ms, which is nearly two times longer than the longest one of “green” QDs ever reported. We believe that the as-synthesized Mn2+-doped CIS-ZnS QDs with high qualities might open new doors for their practical applications in bioimaging and opto/electronic devices.
Methods
Raw materials
The materials of zinc stearate (ZnSt2, ZnO 11.3–11.5%), cuprous chloride (CuCl, 99.999%), sulfur powder (S, 99.99%), 1-dodecanethiol (DDT, 98%), 1-octadecanethiol (ODT, 97%), oleylamine (OLA, 70%), manganese acetate (Mn(Ac)2), and tetrabutylammonium hexafluorophosphate (98%) were commercially available from Aladdin company, China. 1-octadecene (ODE, 90%) was bought from Adamas-beta company, China. Indium (III) acetate (In(Ac)3, 99.99% trace metals basis) was purchased from Aldrich company, China. All the chemicals were used directly without further purification.
Preparation of stock solutions
S stock solutions were prepared by taking 6.4 mg S powders and 0.2 mL OLA into 1.5 mL ODE with gently heating under argon atmosphere. Zinc precursor solutions were prepared using 0.632 g ZnSt2, 2 mL OLA and 2 mL DDT dissolved in 6 mL ODE. A typical manganese precursor solution was prepared by dissolving 5 mg Mn(Ac)2 and 0.5 mL OLA in 1.5 mL ODE. Several types of manganese precursor solutions were prepared for growing Mn2+-doped CuInS-ZnS QDs with tailored doping concentrations.
Synthesis of Mn2+-doped CuInS-ZnS QDs
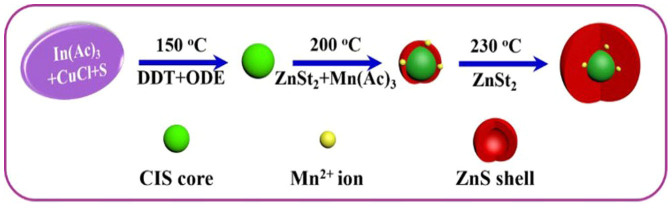
The Mn2+-doped CuInS-ZnS QDs were synthesized by a hot-injection method with following three typical steps (as shown in Figure 1). Firstly, CIS cores were grown. Then the zinc precursors with various amounts of manganese precursor contents were injected into the solutions for the growth of a Zn1−xMnxS shell around the CIS core. Subsequently, the ZnS shells were grown by introducing the stock solutions of zinc precursors, finally leading to the formation of Mn2+-doped CIS-ZnS QDs. In a typical experimental procedure, 1 mg CuCl, 29 mg In(Ac)3, 2 mL DDT and 5 mL ODE were transferred into a 100 mL three necked flask, and degassed for 20 min by bubbling with Ar at 150°C. Then the S stock solution was injected quickly into the flask to form CIS nanoclusters. The solution was then annealed for 10 min, followed by being rapidly injected into 1 mL Zn stock solution and manganese precursor solution. At the meantime, the temperature was heated up to 200°C, and maintained there for 20 min to allow the growth of Zn1−xMnxS shell. After that, the temperature was heated up to 230°C, and 8 mL Zn stock solution was then introduced into the reaction mixture in 4 batches at an interval of 20 min. The resultant colloidal solutions were cooled down naturally to room temperature, followed by purified repeatedly using methanol/hexanes and precipitated using acetone. To make the growth of the Mn2+-doped CIS-ZnS QDs with various Cu/In ratios, the Mn dopant nominal concentration and the In precursor were kept at 2.25 mol% and 0.1 mmol, respectively, with otherwise same experimental procedures. For the synthesis of CIS-ZnS QDs with different Mn doping concentrations, the Cu/In nominal ratios were kept at 1/10 with otherwise same experimental procedures. The experimental results suggested that the production of the QDs via the present multi-step hot-injection strategy was highly repeatable.
Figure 1. Schematic illustration for the growth of Mn2+-doped CIS-ZnS QDs via the multi-step hot-injection strategy.
Structure characterization and optical property measurements
The obtained QDs were characterized using high-resolution transmission electron microscopy (HRTEM, JEM-2100F, JEOL, Japan) equipped with energy dispersive X-ray spectroscopy (EDX, Quantax-STEM, Bruker, Germany), and X-ray diffraction (XRD, D8 Advance, Bruker, Germany). The average diameter of the Mn2+-doped QDs was decided by the Scherrer's law:
 |
Where K is a dimensionless shape factor (a typical value of 0.9 for spherical objects used as the first approximation), β is the full-width at half maxima (FWHM) of the peak, and θ is the Bragg angle of the corresponding diffraction peak.
The UV-Vis measurements of the obtained QDs were performed on a UV-Vis scanning spectrophotometer (U-3900, Hitachi, Japan). The PL spectra, PL QY, and PL decay curves were recorded using a spectrometer (Fluromax-4P, Horiba Jobin Yvon, France) equipped with a quantum-yield accessory and a time-correlated single-photon-counting (TCSPC) spectrometer. A pulsed xenon lamp and NanoLED (wavelength: 370 nm) were utilized as the exciting sources for PL decay measurements. The PL decay curves were analyzed using DAS6 software. The lifetime data was fitted by the biexponential function:
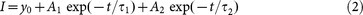 |
Where τ1 and τ2 are the time constants, respectively, A1 and A2 are the normalized amplitudes of the components, respectively. The average lifetime was calculated by:
 |
The electron paramagnetic resonance (EPR) was taken on an X-band spectrometer (EPR, ER200-SRC, Bruker, Germany). The cyclic voltammograms (CV) were recorded on an electrochemical workstation (CHI600, Chenhua, China), using the glassy carbon disc, Pt wire and Ag/AgCl as the working, counter and reference electrodes, respectively. 0.1 M tetrabutylammonium hexafluorophosphate (TBAPF6) dissolved in acetonitrile was employed as the supporting electrolyte. The working electrodes were polished, cleaned, and dried before depositing the NC samples. Then a drop of diluted NC solution was deposited onto the surface of the working electrode to form a NC film. The scan rate was set at 50 mV/s. During all the experiments, the electrolyte solutions were thoroughly deoxygenated by bubbling nitrogen gas (99.99%).
Results
Structure and optical property of Mn2+-doped CIS-ZnS QDs
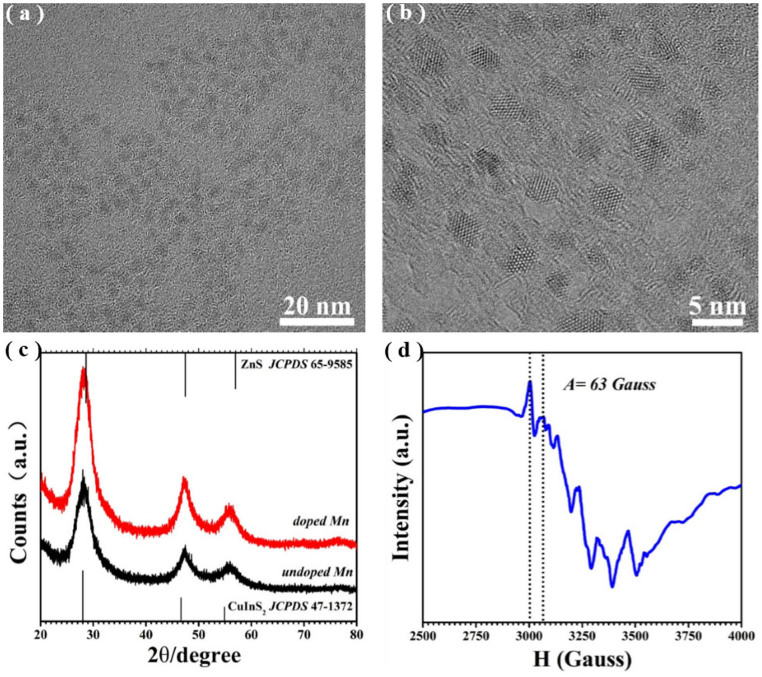
Figure 2(a) shows a typical TEM image of Mn2+-doped CIS-ZnS QDs. The QDs are nearly spherical (see also Supplementary Figure S1(a)), with a fairly monodisperse size distribution (the inset in Supplementary Figure S1(a)). The average size of these QDs is 3.7 ± 0.3 nm, as determined from the TEM images. The lattice fringes are clear (Figure 2(b)), indicating the crystalline nature of the resultant QDs. Figure 2(c) shows the representative X-ray diffraction (XRD) patterns of the CIS-ZnS QDs with and without Mn dopants, indicating no apparent difference between them. The peaks are broadened, implying the tiny size of the resultant QDs. All the diffraction peaks are centered between those of CIS (JCPDS Card No. 471372) and ZnS (JCPDS Card No. 659585), clarifying that the QDs should be zinc blended phase of CIS-ZnS, rather than a mixture of CIS and ZnS. The average diameter of the QDs was ca. 3.6 nm based on the Debye–Scherrer formula by using the (111) diffraction within the XRD pattern, which is in good agreement with the TEM observations. The mean incorporated Mn2+ concentration (i.e., Mn/(Zn + Cu + In)) is ~1.05 mol% from the EDX measurement (see Supplementary Figure S2), which is lower than 2.25 mol% feed in the raw materials. The electron paramagnetic resonance (EPR) spectra also support the presence of Mn2+ in the doped NCs. Figure 2(d) presents a typical EPR spectrum of the as-synthesized Mn2+-doped CIS-ZnS QDs. The hyperfine coupling constant (A) of 63 Gauss calculated from Figure 2(d), is very close to Mn2+ embedded in ZnS NC matrix (64.5 Gauss)29, suggesting that the Mn2+ ions have been successfully incorporated into the QDs. However, the broadening of the peak, rather than the six clear hyperfine splittings, is expected due to the complex environment of the Mn in the presence of copper19,20,21. We also noticed that the dipole-dipole interactions between the Mn2+ impurities can also broaden the EPR signal spectra7, which maybe together give rise to the broadening of the EPR signal spectra.
Figure 2.
(a–b) Typical TEM images of the Mn2+-doped CIS-ZnS QDs under different magnifications. (c) Typical XRD patterns of CIS-ZnS QDs with (red) and without (black) Mn2+ dopants. (d) Typical EPR spectrum of the Mn2+-doped CIS-ZnS QDs by treatment with pyridine.
Figure 3 shows the typical absorption, PL, and PL excitation (PLE) spectra of the doped (i.e., with 2.25 mol% nominal dopants) and undoped CIS-ZnS QDs. The absorption spectra have the similar onset at around 500 nm. The poorly resolved excitonic absorption is ascribed to the typical characteristic of the ternary and quaternary, which is partially caused by the special inhomogeneous composition distributions among different NCs15,16. Unlike the absorption spectra with the similar onset, the emission spectra are different, in which the peaks are centered at ~557 and ~610 nm for the undoped and doped QDs, respectively. The PL in the undoped CIS-ZnS QDs could be attributed to the host defect state emission15,16. The highly intense orange-yellow emission with a PL wavelength of ~610 nm and the FWHM of ~80 nm should result from the 4T1–6A1 transition of the Mn2+ impurity, which is excited by the energy transfer from the host CIS-ZnS, according to the similar onsets of PLE and absorption spectra in Figure 3. The origin of the PL can also be confirmed by the surprisingly long excited-state lifetime (typically in a millisecond time scale) as discussed in the following section (Figure 4)4,10,13,30. What's more, the blue-shifted PL wavelengths in the temperature-dependent PL spectra are also a further indication for the Mn2+ emission. (see Supplementary Figure S3)4,31. Notably, such Mn2+-doped CIS-ZnS QD gives a symmetric red emission centered at 610 nm (2.03 eV), which could be ascribed to the changed crystal field splitting in the coordination environments by the lattice mismatch between CIS cores and ZnS shells14. In addition, the PL FWHM (~80 nm) of these QDs is obviously much wider than that of a single Mn-doped CdS/ZnS QDs (~20 nm)32. This is mainly because that the Mn PL in the current case is an artifact of averaging over a large number of contributions from numerous QDs in different environments32. The PL QY of the host defect state emission is 40% for the undoped counterparts, while that of the Mn2+-doped CIS-ZnS QDs can be up to 66% (the corresponding digital photographs are inset in Figure 3; details for the QY measurement, see Supplementary Figure S4), which is comparable to the best one of Mn2+-doped semiconductor NCs ever reported.
Figure 3. Typical UV-Vis absorption (black), PLE (red), and PL (blue) spectra of the as-synthesized Mn2+-doped (upper) and undoped (below) CIS-ZnS QDs at room temperature.
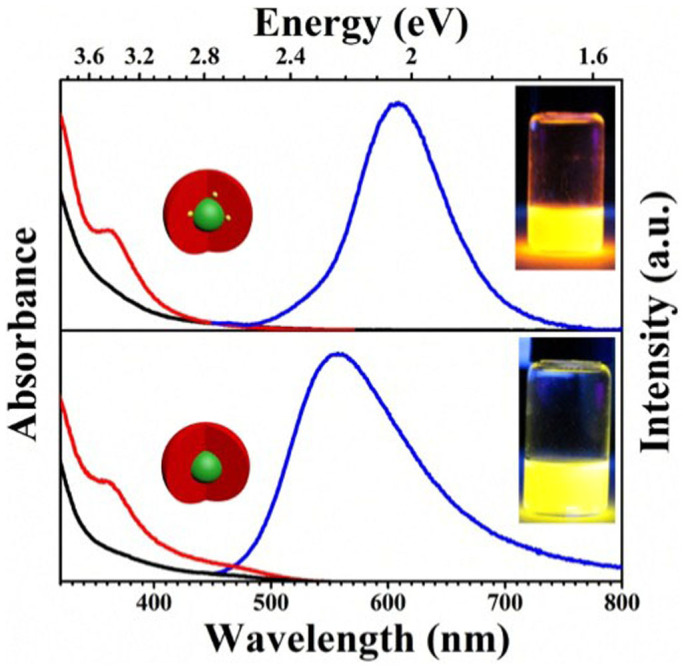
The insets show their corresponding digital photos of these QDs under UV light illumination excited at a wavelength of 365 nm.
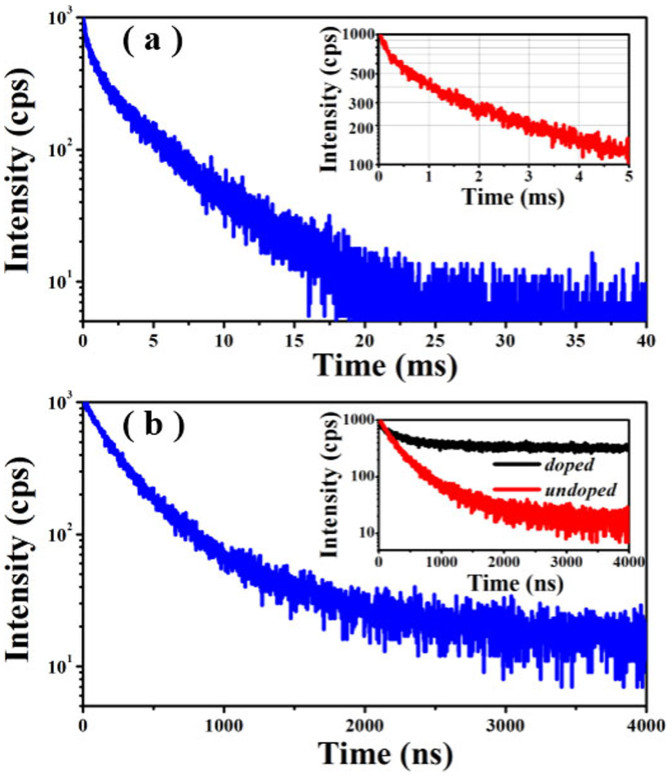
Figure 4.
(a) Typical PL decay spectrum of Mn2+-doped CIS-ZnS QDs. The up-right inset shows the same data in the low time regime. (b) Typical PL decay spectrum of Mn2+-undoped CIS-ZnS QDs. The inset is the PL decay curves of Mn2+-doped and undoped CIS-ZnS QDs in the same time range window.
Figure 4(a) and (b) show the corresponding PL decay curves of the Mn2+-doped and undoped CIS-ZnS QDs at room temperature, respectively. In contrast to undoped QDs with a short PL lifetime often scaled in nanosecond, the Mn2+-doped QDs exhibits a much longer lifetime in a millisecond scale. For the doped CIS-ZnS QDs, to clearly present the decay dynamics, the same data in the low time regime is shown as the inset in Figure 4a. To further compare the decay characteristics between the doped and undoped QDs, their decay curves are plotted in the low time regime from 0 to 4000 ns, which is shown as the inset in Figure 4b. This suggests that the decay of the doped sample is obviously beyond the window of 4000 ns. However, the PL intensity of the undoped counterparts becomes basically invariable after 3000 ns (the residual signals are attributed to the noise). Furthermore, the PL intensity of the undoped QDs decays much quicker than that of the doped sample. These experimental results suggest that there is not obvious overlapping of surface states with the Mn emission in the doped sample. These PL decay lines are fitted by a biexponential function, and their time constants and normalized amplitudes are summarized in the Table 1, respectively. As seen in this table, the average lifetimes are 3.78 ms and 401 ns for doped and undoped QDs, respectively. To the best of our knowledge, such excited-state lifetime of ~3.78 ms is a new record of the longest one for all the “green” Mn2+-doped QDs, which is nearly two times longer than the longest one previously reported (i.e., ~2.12 ms19).
Table 1. Fitting parameters of PL dynamics of Mn2+ doped and undoped CIS-ZnS QDs.
| Sample | A1 | τ1 | A2 | τ1 | χ2 | τave |
|---|---|---|---|---|---|---|
| Mn-doped | 0.576 | 0.62 ms | 0.424 | 4.39 ms | 1.102 | 3.78 ms |
| undoped | 0.785 | 198 ns | 0.215 | 700 ns | 1.036 | 401 ns |
Effect of the Cu/In ratios on the Mn2+ emission in Mn2+-doped CIS-ZnS QDs
Figure 5 shows the evolution of PL spectra of Mn2+-doped CIS-ZnS QDs starting from the CIS cores (the nominal Mn doping concentration and In precursor are kept constants at 2.25 mol% and 0.1 mmol, respectively), which were prepared with the Cu/In nominal molar ratios of 1/2, 3/10, and 1/10, corresponding to Figure 5(a), (b) and (c), respectively. As seen in Figure 5(a) and (b) with the Cu/In nominal molar ratios of 1/2 and 3/10, the overgrowth with Zn1−xMnxS and/or ZnS shell typically leads to a systematical blue shift of the PL spectra, which likely indicates the etching of the core material under the shell growth conditions and associated increase in the degree of the spatial confinement12,16,33. Nonetheless, even the Mn2+ ions have been incorporated into the NCs, it should be noted that there is no detectable Mn2+ emission, since their PL lifetimes scaled in nanosecond (see Supplementary Figure S5). However, for the sample with a reduced Cu/In nominal molar ratio of 1/10 (Figure 5(c)), after the ZnS shell deposition, the well-resolved Mn2+ emission could be achieved. The PL peak wavelengths cannot be further tuned although its intensity is still enhanced with the reactions progressed. In addition, the PL spectra are relatively narrower in contrast to those of the samples with higher Cu/In nominal ratios of 1/2 and 3/10. These observations clearly indicate that the Cu/In ratios play a key role for obtaining the well-resolved Mn2+ d-d emission in the CIS-ZnS QDs. As we known, the decrease of the Cu/In molar ratio will increase the bandgap of CIS cores since In2S3 (2.07 eV) and InS (2.44 eV)) have wider bandgaps than Cu2S (1.2 eV)34,35, which are also confirmed by the blue shifts of the absorption band edge (see Supplementary Figure S6) with a reduced Cu/In ratio and the cyclic voltammetry (C-V) investigations (see Supplementary Figure S7). The host bandgaps are estimated to be ~2.0, 2.1 and 2.3 eV from the absorption and C-V lines for the Mn2+-doped CIS-ZnS QDs grown with the Cu/In nominal ratios of 1/2, 3/10, and 1/10, respectively. Considering the fact that Mn2+ emission can only occur in the QDs with a wider host bandgap over than the Mn2+ ligand field transition energy36,37, this suggests that the host bandgap can reach or exceed the Mn2+ ligand field transition energy (~2.03 eV for our Mn2+-doped CIS-ZnS QDs) with the decreased Cu/In ratio. This might clarify the absence of Mn2+ emission from the Mn2+-doped CIS-ZnS QDs in the reported works, since the Cu/In molar ratios were generally much higher than 1/1021,23,26. We also noticed that, for Mn and Cu co-doped QDs, the Cu emission is dominant with suppressing Mn-related emission when the distance between Cu and Mn ions is close enough38. This accounts for the suppression of the Mn2+ emission in the sample with the Cu/In nominal ratio of 3/10, since the bandgap is marginally larger than the Mn2+ ligand field transition energy. In other words, the Cu/In molar ratios play a key role for obtaining the Mn2+ d-d emission, and a reduced Cu/In molar ratio favors the presence of the well-resolved Mn2+ emission in CIS-ZnS QDs.
Figure 5.
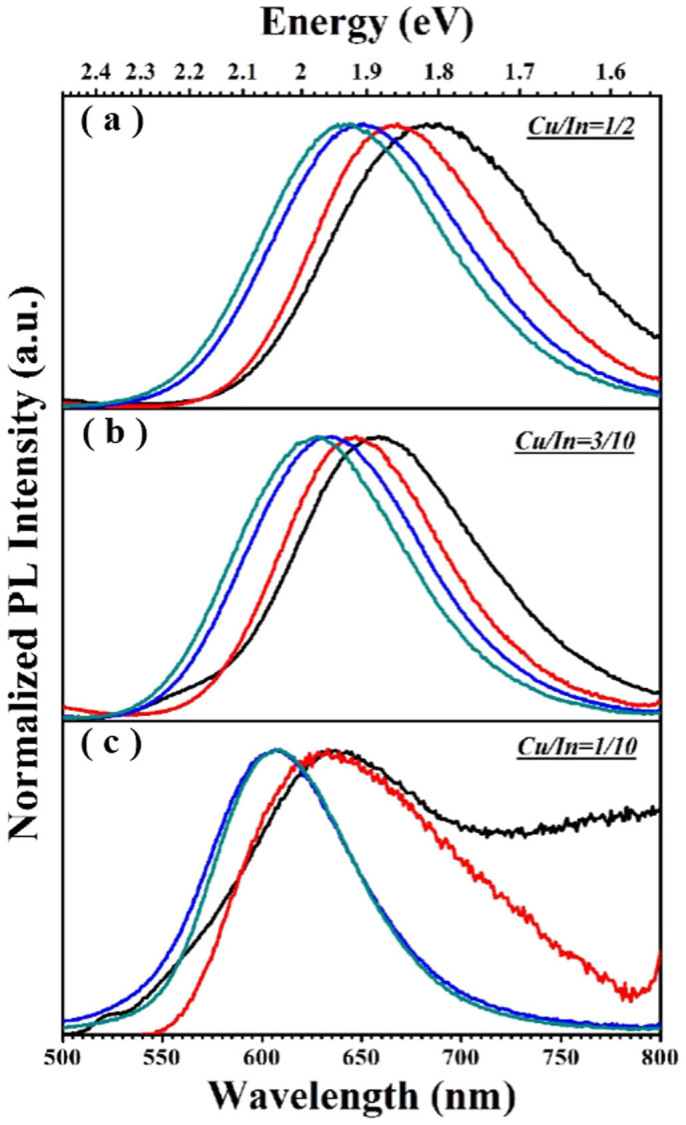
(a–c) Evolution of the PL spectra of Mn2+-doped CIS-ZnS QDs with the overcoating of ZnS shells. The black lines in (a–c) refer to the PL spectrum evolutions of the CIS cores fabricated at the Cu/In ratios of 1/2, 3/10 and 1/10, respectively; The red lines in (a–c) refer to the PL spectrum evolutions of the QDs after the growth of a Zn1−xMnxS layer around the CIS core by injecting the zinc + manganese precursor contents (0.1 mmol ZnSt2 + 0.03 mmol Mn(Ac)2); The blue and cyan lines refer to the PL spectrum evolutions of the QDs after the growth of ZnS shells by introducing zinc precursors of 0.2 ZnSt2 and 0.8 mmol ZnSt2, respectively.
Effect of the Mn2+ doping concentrations on the spectral characteristics in Mn2+-doped CIS-ZnS QDs
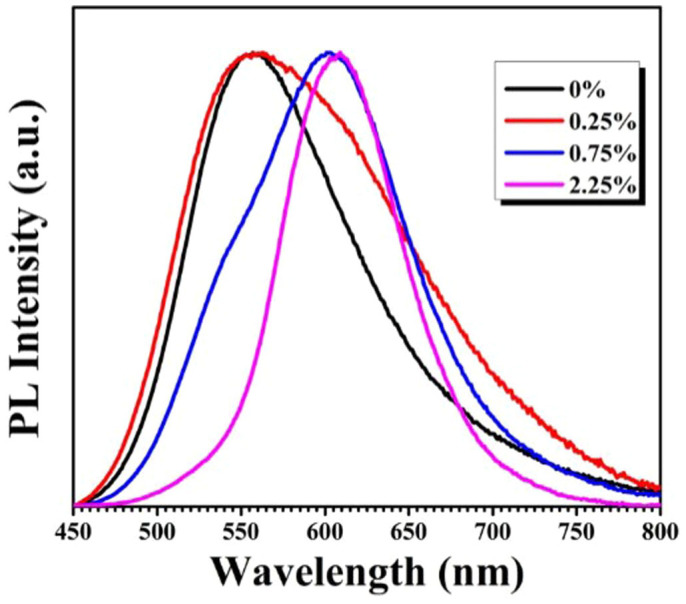
Figure 6 presents the effects of the nominal Mn2+ doping concentrations (i.e. 0–2.25 mol%) on the spectral characteristics of CIS-ZnS QDs with the Cu/In nominal molar ratio kept at 1/10. The FWHM of the PL spectrum becomes narrower with the increase of the Mn2+ doping concentrations, suggesting that the wide band PL of the QDs with a low Mn2+ concentration maybe originates from both host defect state emission and dopant Mn2+ emission. The time-resolved spectroscopy measurements are undertaken to distinguish the origin of the emission for the QDs (see Supplementary Figure S8). The obviously narrowed PL spectrum after the delay for the QDs with a lower Mn2+ concentration (i.e. 0.75 mol%) confirms the combined origin of the PL. Meanwhile, the similar normalized time-resolved PL spectra with various delay times for the sample with a higher Mn concentration (i.e. 2.25 mol%) disclose that the doped QDs possess a basically pure Mn2+ d-d emission. The experimental results imply that the emission in the present QDs can be controlled by tailoring the amounts of Mn dopants7,8,39. The increased Mn concentration will result in the decreased proportion of the host defect state emission in the PL spectra, and the well-resolved Mn2+ d-d emission can be obtained at a nominal doping concentration of 2.25% in our case.
Figure 6. Representative PL spectra of Mn2+ doped CIS-ZnS QDs with different Mn initial concentrations at an excitation wavelength of 365 nm.
Discussion
The growth of CIS-ZnS QDs in current work with a core-shell structure, rather than a homogeneous CZIS alloyed one and other side binary product, could be supported by following issues:
From the point of synthesis procedure. As reported in the previous works, the homogeneous alloyed QDs were often obtained via a one-pot method that all of precursors are introduced together at one time17,40. Different from the one-pot route, the multi-step hot-injection strategy (similar to our case, see the Experimental Procedure) often favors the growth of the QDs with a unique core-shell structure. For example, Park et al. have detailedly studied the overgrowth process of ZnS shell on CuInS2 cores to understand the obtained structure of CIS-ZnS QDs, which concluded that the CIS cores are not homogeneous alloy but a gradient composition after overcoated ZnS shell, namely the typical core-shell structures41. In our case, the temperature for the overgrowth of ZnS shell is identical to that in the literature as mentioned above (i.e. 230°C), which leads to the formation of the CIS-ZnS QDs in a typical core-shell structure41,42,43,44,45. After growth of the ZnS shells, the diameter of the CIS/Zn1−xMnxS QDs is increased from 2.8 ± 0.3 nm (Figure S1(b)) to 3.7 ± 0.3 nm. Meanwhile, the corresponding XRD pattern also showed an obvious peak shift (see Figure S9, Supplementary Information). These facts indicate that the ZnS shells have been overcoated on the CIS/Zn1−xMnxS cores. Thus, we deduced that, in the present work, the growth of CIS-ZnS QDs should be in a core-shell structure rather than a homogeneous alloyed one.
From the point of band gap. According to the reported work of alloyed (CuInS2)x(ZnS)1−x NCs by Pan et al., the band gaps of these alloyed NCs with zinc blended phase can be tuned in the broad range from 1.5 to 3.7 eV by changing the ratios of CuInS2 to ZnS17. In our case, the Cu/In/Zn mole ratio is ~1/4/30 (see Figure S2, Supplementary Information). If the homogeneous alloy structures were formed, the bandgap of the doped-QDs should be ~3.3 eV. However, the host bandgap is estimated to be 2.3 eV from the absorption and C-V lines of our QDs, suggesting that our obtained QDs are not a homogeneous alloy structure.
From the PL spectral characteristics. It has been proposed that the preparation of CIS-ZnS QDs has a great challenge to balance the reactivity of the metallic cations each other, in which the unfavorable phase separation will bring a mixture of binary products. Usually, for the Mn2+-doped QDs, the Mn2+ emission is very sensitive to the located matrix environments13,46, which offers us a method to confirm the main products of the as-synthesized samples. Figure 7 shows the two-dimensional PL excitation (2d PLE) spectra of the synthesized QDs, which is obtained by measuring the PL spectra over a range of excitation energies containing the information of PLE and PL spectra. As shown in Figure 7, these Mn2+-doped QDs can be excited even with 440 nm wavelength light, indicating that the Mn2+ d-d emission should be mainly excited by the energy transfer from the target multiple CIS-ZnS host, rather than some possible side products such as Mn:ZnS (at least not completely, since the bandgap of ZnS (~3.7 eV) is much larger than the excitation onset of the QDs). Furthermore, the PL spectra possess basically similar peak wavelengths at ~610 nm when excited at the wavelengths ranged from 320 to 460 nm, which indicate that the local environment of emission centers should be homogeneous CIS-ZnS host rather than ZnS QDs, since the PL of the Mn:ZnS QDs is generally centered at ~580 nm. In addition, the PLE spectra (see Supplementary Figure S10) for the Mn2+-doped QDs taken at the different emission peak wavelengths are overlapped with each other, implying that the emission originates from the same particle set rather than from the extraneous species or undesired secondary formed QDs during the synthesis procedure. Thus, it could be confirmed that the as-synthesized product is Mn2+-doped CIS-ZnS QDs rather than any other side binary products.
Figure 7. Representative 2d PLE spectra of Mn2+-doped CIS-ZnS QDs.
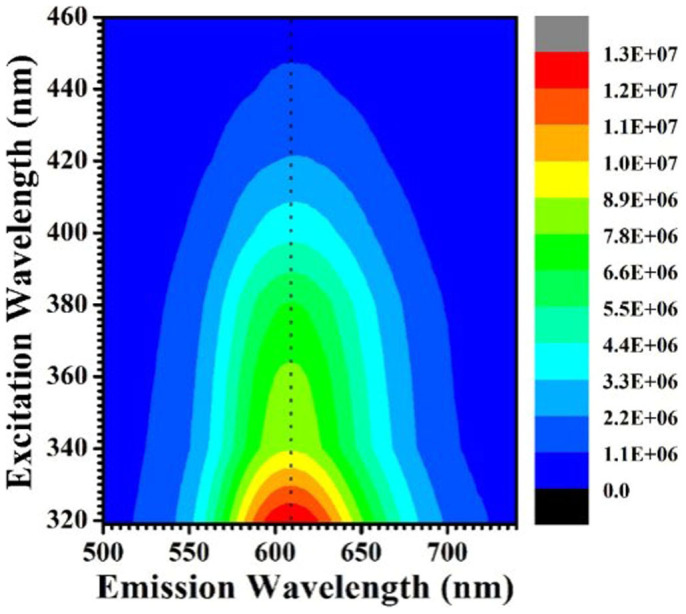
The PL intensities are represented by the colors.
It is widely considered that a long excited state lifetime is one of the key factors for the design and development of the QDs nanodevices (e.g., bioimaging and QDSC)3,22,27. A longer excited-state carrier lifetime can not only facilitate the charge separation in QDSC, but also favor to eliminating the signal interference from the short life material in bioimaging. Table 2 summarizes the state-of-the-art Mn2+ doped QDs in the reported works, suggesting that most of their PL QYs are typically lower than 60%. Furthermore, it should be worthwhile that these Mn2+ doped QDs hosts either require high excitation energies (>3.6 eV), or contain the toxic elements such as Cd and/or Se, which are listed as a Class A elements against the environment-friendly applications. However, our Mn2+-doped CIS-ZnS core-shell QDs have a narrower host bandgap without toxic element Cd and/or Se. Most importantly, besides to their high PL QY up to 66% and well-resolved Mn2+ emission, the present CIS-ZnS QDs possess an extremely long excited state lifetime up to 3.78 ms, which is the longest one ever reported. This is mainly attributed to the high-quality of CIS-ZnS QDs with a favorable Cu/In ratio and Mn2+ doping concentration.
Table 2. Optical properties of Mn-doped QDs in the reported works.
Conclusions
In summary, we have demonstrated the growth of high-quality Mn2+-doped CIS-ZnS QDs based on a multi-step hot-injection method. The Cu/In molar ratios play a key role for obtaining the Mn2+ d-d emission, and a reduced Cu/In molar ratio favors the well-resolved Mn2+ emission in CIS-ZnS QDs. The increased Mn concentration will result in the decreased proportion of the host defect-trap emission in the PL spectra, and the well-resolved Mn2+ d-d emission can be obtained at a favorable doping concentration. The resultant CIS-ZnS QDs exhibit a well-resolved Mn2+ d-d emission with a high PL QY up to 66% and an extremely long excited state lifetime up to ~3.78 ms, which is nearly two times longer than the longest one of “green” QDs ever reported. It is believed that the as-fabricated “green” QDs in current work, with such extremely-long radiative excited-state lifetime and high efficiency, could be an excellent candidate for the practical applications in biological/biomedicine and opto/electronic devices.
Supplementary Material
SI_revised
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (NSFC, Grant No. 61106066), 973 Program (Grant No. 2012CB326407) and Zhejiang Provincial Science Foundation (Grant No. LY14F040001).
Footnotes
The authors declare no competing financial interests.
Author Contributions J.Z., C.L. and W.Y. conceived and directed the experiments. S.C., L.W., M.S. and G.W. performed the experiments. S.C., J.Z. and W.Y. co-wrote the manuscript. All authors discussed the results and helped with the preparation of the final manuscript.
References
- Beaulac R., Schneider L., Archer P. I., Bacher G. & Gamelin D. R. Light-induced spontaneous magnetization in doped colloidal quantum dots. Science 325, 973–976 (2009). [DOI] [PubMed] [Google Scholar]
- Dai Q., Sabio E. M., Wang W. & Tang J. Pulsed laser deposition of Mn doped CdSe quantum dots for improved solar cell performance. Appl. Phys. Lett. 104, 183901 (2014). [Google Scholar]
- Wu P. & Yan X. P. Doped quantum dots for chemo/biosensing and bioimaging. Chem. Soc. Rev. 42, 5489–5521 (2013). [DOI] [PubMed] [Google Scholar]
- Yuan X. et al. Thermal stability of Mn2+ ion luminescence in Mn-doped core-shell quantum dots. Nanoscale 6, 300–307 (2014). [DOI] [PubMed] [Google Scholar]
- Bhargava R., Gallagher D., Hong X. & Nurmikko A. Optical properties of manganese-doped nanocrystals of ZnS. Phys. Rev. Lett. 72, 416–419 (1994). [DOI] [PubMed] [Google Scholar]
- Sapra S., Prakash A., Ghangrekar A., Periasamy N. & Sarma D. Emission properties of manganese-doped ZnS nanocrystals. J. Phys. Chem. B 109, 1663–1668 (2005). [DOI] [PubMed] [Google Scholar]
- Nag A. et al. A study of Mn2+ doping in CdS nanocrystals. Chem. Mater. 19, 3252–3259 (2007). [Google Scholar]
- Nag A., Chakraborty S. & Sarma D. To dope Mn2+ in a semiconducting nanocrystal. J. Am. Chem. Soc. 130, 10605–10611 (2008). [DOI] [PubMed] [Google Scholar]
- Acharya S., Sarma D. D., Jana N. R. & Pradhan N. An alternate route to high-quality ZnSe and Mn-doped ZnSe nanocrystals. J. Phys. Chem. Lett. 1, 485–488 (2010). [Google Scholar]
- Zheng J. et al. Improved photoluminescence of MnS/ZnS core/shell nanocrystals by controlling diffusion of Mn ions into the ZnS shell. J. Phys. Chem. C 114, 15331–15336 (2010). [Google Scholar]
- Zeng R., Zhang T., Dai G. & Zou B. Highly emissive, color-tunable, phosphine-free Mn:ZnSe/ZnS core/shell and Mn:ZnSeS shell-alloyed doped nanocrystals. J. Phys. Chem. C 115, 3005–3010 (2011). [Google Scholar]
- Zhang W., Li Y., Zhang H., Zhou X. & Zhong X. Facile synthesis of highly luminescent Mn-doped ZnS nanocrystals. Inorg. Chem. 50, 10432–10438 (2011). [DOI] [PubMed] [Google Scholar]
- Cao S. et al. Highly efficient and well-resolved Mn2+ ion emission in MnS/ZnS/CdS quantum dots. J. Mater. Chem. C 1, 2540–2547 (2013). [Google Scholar]
- Hazarika A., Pandey A. & Sarma D. D. Rainbow Emission from an Atomic Transition in Doped Quantum Dots. J. Phys. Chem. Lett. 5, 2208–2213 (2014). [DOI] [PubMed] [Google Scholar]
- Aldakov D., Lefrançois A. & Reiss P. Ternary and quaternary metal chalcogenide nanocrystals: synthesis, properties and applications. J. Mater. Chem. C 1, 3756–3776 (2013). [Google Scholar]
- Zhong H., Bai Z. & Zou B. Tuning the luminescence properties of colloidal I-III-VI semiconductor nanocrystals for optoelectronics and biotechnology applications. J. Phys. Chem. Lett. 3, 3167–3175 (2012). [DOI] [PubMed] [Google Scholar]
- Pan D. et al. Alloyed semiconductor nanocrystals with broad tunable band gaps. Chem. Commun. 28, 4221–4223 (2009). [DOI] [PubMed] [Google Scholar]
- Beaulac R. et al. Spin-polarizable excitonic luminescence in colloidal Mn2+-doped CdSe quantum dots. Nano Lett. 8, 1197–1201 (2008). [DOI] [PubMed] [Google Scholar]
- Manna G., Jana S., Bose R. & Pradhan N. Mn-doped multinary CIZS and AIZS nanocrystals. J. Phys. Chem. Lett. 3, 2528–2534 (2012). [DOI] [PubMed] [Google Scholar]
- Liu Q., Deng R., Ji X. & Pan D. Alloyed Mn-Cu-In-S nanocrystals: a new type of diluted magnetic semiconductor quantum dots. Nanotechnology 23, 255706 (2012). [DOI] [PubMed] [Google Scholar]
- Lin B. et al. Multifunctional manganese-doped core-shell quantum dots for magnetic resonance and fluorescence imaging of cancer cells. New J. Chem. 37, 3076–3083 (2013). [Google Scholar]
- Luo J. et al. Highly efficient core-shell CuInS2-Mn doped CdS quantum dot sensitized solar cells. Chem. Commun. 49, 3881–3883 (2013). [DOI] [PubMed] [Google Scholar]
- Hua J. et al. Photoluminescence properties of Cu-Mn-In-S/ZnS core/shell quantum dots. Superlattices Microstruct. 73, 214–223 (2014). [Google Scholar]
- Sitbon G. et al. Multimodal Mn-doped I-III-VI quantum dots for near infrared fluorescence and magnetic resonance imaging: from synthesis to in vivo application. Nanoscale 6, 9264–9272 (2014). [DOI] [PubMed] [Google Scholar]
- Yang Y., Chen O., Angerhofer A. & Cao Y. C. Radial-position-controlled doping in CdS/ZnS core/shell nanocrystals. J. Am. Chem. Soc. 128, 12428–12429 (2006). [DOI] [PubMed] [Google Scholar]
- Ding K., Jing L., Liu C., Hou Y. & Gao M. Magnetically engineered Cd-free quantum dots as dual-modality probes for fluorescence/magnetic resonance imaging of tumors. Biomaterials 35, 1608–1617 (2014). [DOI] [PubMed] [Google Scholar]
- Santra P. K. & Kamat P. V. Mn-doped quantum dot sensitized solar cells: a strategy to boost efficiency over 5%. J. Am. Chem. Soc. 134, 2508–2511 (2012). [DOI] [PubMed] [Google Scholar]
- Lin J. et al. Atomically precise doping of monomanganese ion into coreless supertetrahedral chalcogenide nanocluster inducing unusual red shift in Mn2+ emission. J. Am. Chem. Soc. 136, 4769–4779 (2014). [DOI] [PubMed] [Google Scholar]
- Yang B., Shen X., Zhang H., Cui Y. & Zhang J. Luminescent and magnetic properties in semiconductor nanocrystals with radial-position-controlled Mn2+ doping. J. Phys. Chem. C 117, 15829–15834 (2013). [Google Scholar]
- Zheng J. et al. Efficient photoluminescence of Mn2+ ions in MnS/ZnS core/shell quantum dots. J. Phys. Chem. C 113, 16969–16974 (2009). [Google Scholar]
- Zheng J. et al. Temperature-dependent photoluminescence properties of Mn:ZnCdS quantum dots. RSC Adv. 4, 30948–30952 (2014). [Google Scholar]
- Hazarika A. et al. Ultranarrow and widely tunable Mn2+-induced photoluminescence from single Mn-doped nanocrystals of ZnS-CdS alloys. Phys. Rev. Lett. 110, 267401 (2013). [DOI] [PubMed] [Google Scholar]
- Chen B. et al. Highly emissive and color-tunable CuInS2-based colloidal semiconductor nanocrystals: off-stoichiometry effects and improved electroluminescence performance. Adv. Funct. Mater. 22, 2081–2088 (2012). [Google Scholar]
- Kim Y. et al. Synthesis of Au-Cu2S core-shell nanocrystals and their photocatalytic and electrocatalytic activity. J. Phys. Chem. C 114, 22141–22146 (2010). [Google Scholar]
- Khanchandani S., Kundu S., Patra A. & Ganguli A. K. Band Gap Tuning of ZnO/In2S3 core/shell nanorod arrays for enhanced visible-light-driven photocatalysis. J. Phys. Chem. C 117, 5558–5567 (2013). [Google Scholar]
- Pradhan N. & Sarma D. Advances in light-emitting doped semiconductor nanocrystals. J. Phys. Chem. C Lett. 2, 2818–2826 (2011). [Google Scholar]
- Beaulac R., Archer P. I., Ochsenbein S. T. & Gamelin D. R. Mn2+-doped CdSe quantum dots: new inorganic materials for spin-electronics and spin-photonics. Adv. Funct. Mater. 18, 3873–3891 (2008). [Google Scholar]
- Panda S. K., Hickey S. G., Demir H. V. & Eychmuller A. Bright white-light emitting manganese and copper co-doped ZnSe quantum dots. Angew. Chem. Int. Ed. 50, 4432–4436 (2011). [DOI] [PubMed] [Google Scholar]
- Yang H. & Holloway P. H. Enhanced photoluminescence from CdS:Mn/ZnS core/shell quantum dots. Appl. Phys. Lett. 82, 1965–1967 (2003). [Google Scholar]
- Zhang J., Xie R. & Yang W. A simple route for highly luminescent quaternary Cu-Zn-In-S nanocrystal emitters. Chem. Mater. 23, 3357–3361 (2011). [Google Scholar]
- Park J. & Kim S. W. CuInS2/ZnS core/shell quantum dots by cation exchange and their blue-shifted photoluminescence. J. Mater. Chem. 21, 3745–3750 (2011). [Google Scholar]
- Pons T. et al. Cadmium-free CuInS2/ZnS quantum dots for sentinel lymph node imaging with reduced toxicity. ACS Nano 4, 2531–2538 (2010). [DOI] [PubMed] [Google Scholar]
- Li L. et al. Highly luminescent CuInS2/ZnS core/shell nanocrystals: cadmium-free quantum dots for in vivo imaging. Chem. Mater. 21, 2422–2429 (2009). [Google Scholar]
- Guo W. Synthesis of Zn-Cu-In-S/ZnS core/shell quantum dots with inhibited blue-shift photoluminescence and applications for tumor targeted bioimaging. Theranostics 3, 99 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng D. et al. High-quality CuInS2/ZnS quantum dots for in vitro and in vivo bioimaging. Chem. Mater. 24, 3029–3037 (2012). [Google Scholar]
- Pradhan N., Battaglia D. M., Liu Y. & Peng X. Efficient, stable, small, and water-soluble doped ZnSe nanocrystal emitters as non-cadmium biomedical labels. Nano Lett. 7, 312–317 (2007). [DOI] [PubMed] [Google Scholar]
- Srivastava B. B. et al. Highly luminescent Mn-doped ZnS nanocrystals: gram-scale synthesis. J. Phys. Chem. Lett. 1, 1454–1458 (2010). [Google Scholar]
- Menkara H. et al. Development of nanophosphors for light emitting diodes. Opt. Express 19, A972–A981 (2011). [DOI] [PubMed] [Google Scholar]
- Weaver A. L. & Gamelin D. R. Photoluminescence brightening via electrochemical trap passivation in ZnSe and Mn2+-doped ZnSe quantum dots. J. Am. Chem. Soc. 134, 6819–6825 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SI_revised