Figure 1.
PHD2 Induces Ubiquitination and Degradation of B55α
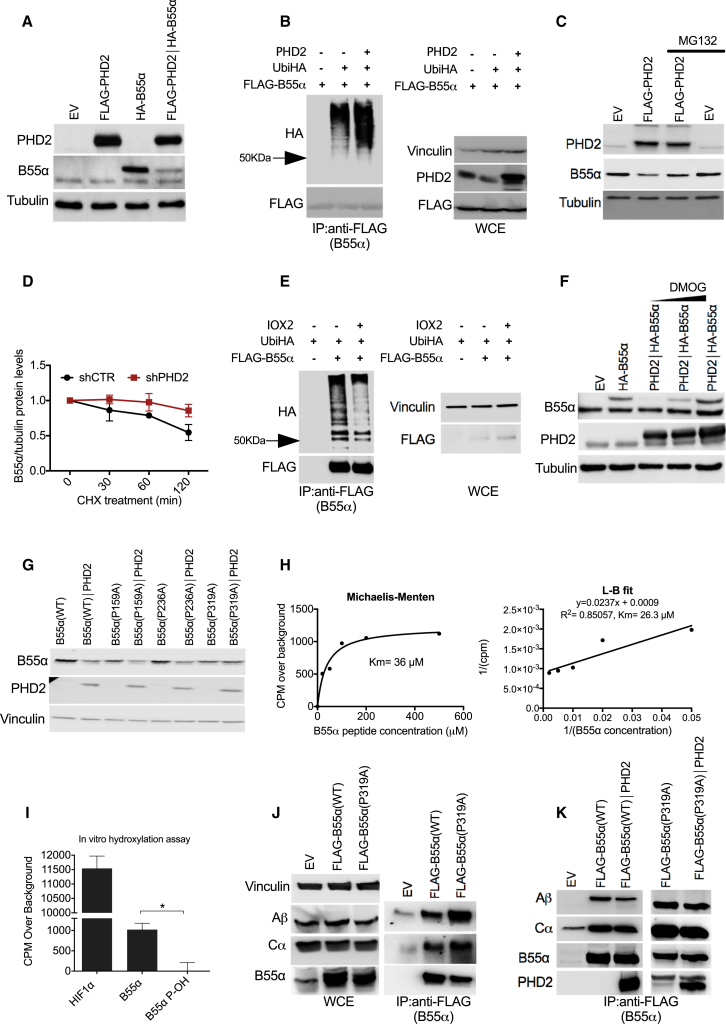
(A) HEK293T cells were transfected with plasmids encoding for nothing (EV [empty vector]), B55α, PHD2, or both. After 24 hr, whole-cell extracts (WCEs) were lysed and analyzed by western blot (WB).
(B) HEK293T cells were transfected with FLAG-B55α alone or in combination with plasmids carrying Ubiquitin-HA and with PHD2 or a control vector. After 16 hr, cells were treated with MG132 (10 μM) for 4 hr, lysed, and immunoprecipitated using anti-FLAG M2 beads to detect Ubiquitin-HA. WB on WCEs is shown on the right.
(C) WB analysis of HEK293T cells transfected with B55α alone or in combination with PHD2 and treated with MG132 (10 μM) or vehicle for 8 hr.
(D) HEK293T cells stably expressing a shRNA specifically targeting PHD2 (shPHD2) or control (shCTR) were transfected with B55α. Then, cells were treated with 100 μg/mL cycloheximide for the indicated time points; WCEs were collected and analyzed by WB. The graph represents the quantification of three independent experiments.
(E) HEK293T cells were transfected with Ubiquitin-HA alone or in combination with FLAG-B55α or control vector. After 8 hr, cells were treated with the PHD2 inhibitor IOX2 (50 μM) for 16 hr and immunoprecipitated using anti-FLAG M2 beads to detect Ubiquitin-HA. WB on WCEs is shown on the right.
(F) HEK293T cells were transfected with B55α alone or in combination with PHD2. After 24 hr, cells were incubated for 8 hr in the presence or absence of the PHD2 inhibitor DMOG (1 or 2 mM). Protein levels were analyzed by WB.
(G) WB analysis of HEK293T cells stably transfected with plasmids carrying wild-type B55α (WT) or B55α mutants (P159A, P236A, P319A) in the presence or absence of PHD2.
(H) In vitro hydroxylation assays were performed with increasing concentrations of a B55α peptide (spanning from W311 to L329). Km was determined by using Michaelis-Menten curve (left panel) or L-B plot (right panel).
(I) In vitro hydroxylation assays were performed on three different peptides: HIF1α (556–575), B55α (311–329), and B55α P-OH (where P319 is hydroxylated). ∗p < 0.05 versus the other conditions. The graph shows the mean ± SEM.
(J) HEK293T cells were transfected with either empty vector (EV), FLAG-B55αWT, or FLAG-B55αP319A. After 16 hr, cells were lysed and immunoprecipitated using anti-FLAG M2 beads to detect the A and C subunits of the complex PP2A. WB on whole-cell extracts (WCEs) is shown on the left.
(K) HEK293T cells were transfected with either empty vector (EV), FLAG-B55αWT, or FLAG-B55αP319A. Cell lysates expressing FLAG-B55αWT or FLAG-B55αP319A were incubated in vitro for 2 hr with recombinant PHD2. Then an anti-FLAG immunoprecipitation was performed in order to detect by WB the levels of the Ab subunit as readout of PP2A complex information.
See also Figure S1.