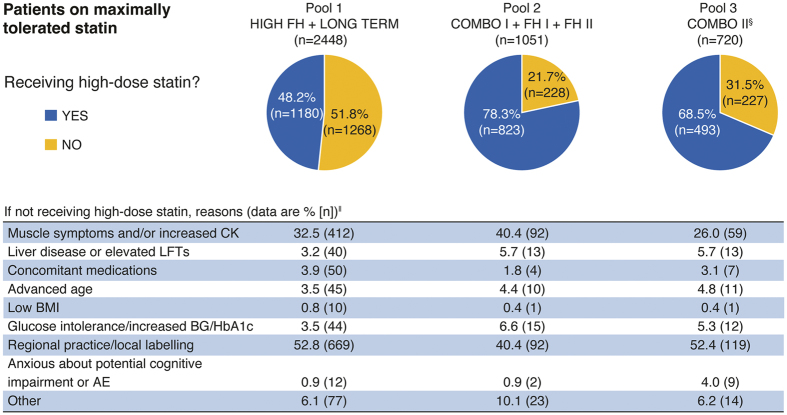
Figure 2. Investigator-approved reasons why patients were not receiving a high-dose statin† in studies requiring participants to be on maximally tolerated statin‡.
†High dose statin defined as: atorvastatin 40–80 mg, rosuvastatin 20–40 mg, or simvastatin 80 mg. ‡All patients in Pool 1 and 2 and patients from COMBO II in Pool 3 were required to be on maximally tolerated statin at study entry, ideally a high-dose statin although lower doses were allowed with an investigator-approved reason. §OPTIONS I and II not included as patients received study-defined doses of background statin rather than maximally tolerated doses. ||A patient can be counted in several categories. AE, adverse event; BG, blood glucose; BMI, body mass index; CK, creatine kinase; HbA1c, glycated haemoglobin; LFT, liver function test.