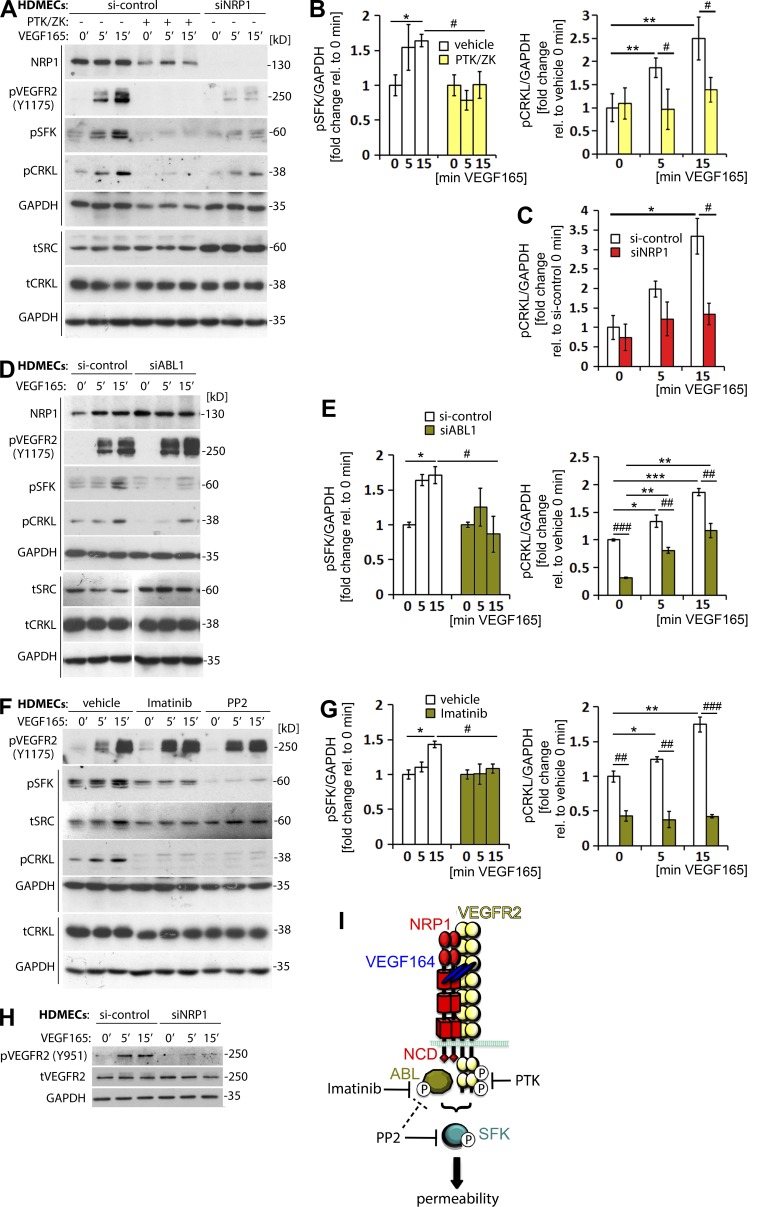
Figure 5.
VEGFR2 and NRP1 are required for VEGF165-induced SFK activation via ABL1. (A–C) Confluent HDMEC cultures transfected with si-control or siNRP1 were serum-starved and treated with VEGF165 for the indicated times. Cultures were also treated with vehicle (-) or PTK/ZK (+) for 30 min before VEGF165 stimulation. Lysates were used for immunoblotting with the indicated antibodies (A), followed by quantification of pSFK levels (B, left) and pCRKL levels (B’, right, and C) relative to GAPDH (four independent experiments). Each of the two vertical lines indicated a group of immunoblots from a single gel, with both gels containing aliquots of the same protein lysate. (D–E) Confluent HDMEC cultures transfected with si-control or siABL1 were serum-starved and treated with VEGF165 for the indicated times. Lysates were used for immunoblotting with the indicated antibodies (D), followed by quantification of pSFK levels (E, left) and pCRKL levels (E’, right) relative to GAPDH (four independent experiments). Each of the two vertical lines indicates a group of immunoblots from a single gel, with both gels containing aliquots of the same protein lysate. The spacer line (D, bottom) separates lanes 4–6 (left) from lanes 1–3 (right) of immunoblots from the gel in Fig. S1. (F–G) Confluent HDMEC cultures were serum-starved and treated with vehicle, Imatinib or PP2 for 30 min before VEGF165 stimulation for the indicated times. Lysates were used for immunoblotting with the indicated antibodies (F), followed by quantification of pSFK levels (G, left) and pCRKL levels (G’, right) relative to GAPDH (three independent experiments). Each of the two vertical lines indicates a group of immunoblots obtained from a single gel, with both gels containing aliquots of the same protein lysate. In B, E, and G (left) data are expressed as fold change, mean ± SEM, in VEGF165-treated cells at 5 and 15 min relative to 0 min; in C and B, E, and G (right), data are expressed as fold change, mean ± SEM, in VEGF165-treated cells at 5 and 15 min relative to control cells at 0 min; asterisks indicate P-values for induction after VEGF165 treatment (*, P < 0.05; **, P < 0.01; ***, P < 0.001; paired Student’s t test); hash tags indicate significant P-values for different treatments at corresponding time points (#, P < 0.05; ##, P < 0.01; ###, P < 0.001; unpaired Student’s t test; n ≥ 3 independent experiments). (H) Confluent HDMEC cultures transfected with si-control or siNRP1 and serum-starved were treated with VEGF165 for the indicated times and lysates used for immunoblotting with the indicated antibodies (three independent experiments). (I) Model for VEGF165-induced vascular permeability signaling including the point of interference by pharmacological inhibitors used in this study.