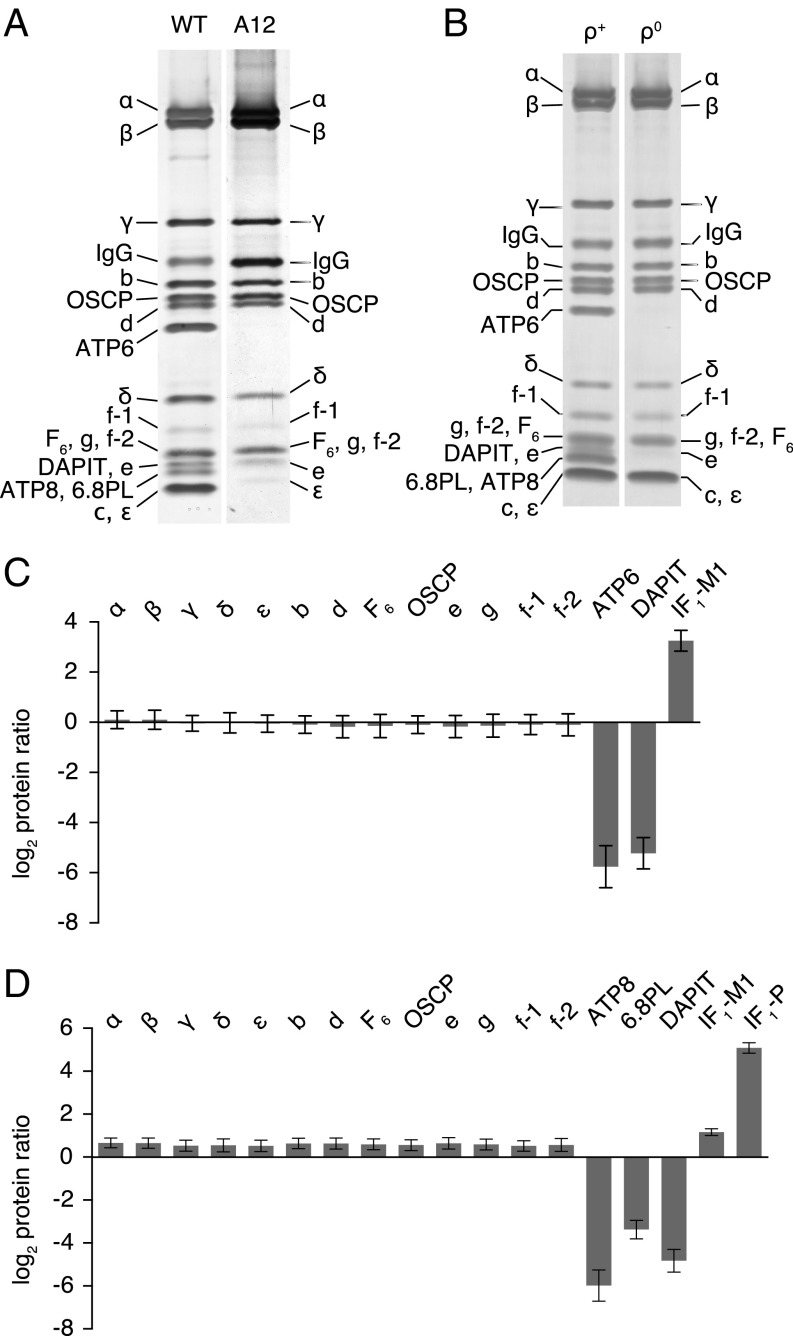
Fig. 4.
Effects of the deletion of the c-subunit of human ATP synthase in HAP1-A12 cells and of the removal of ATP6 and ATP8 in ρ0 cells. (A) Impact of removal of c-subunits on the subunit composition of the vestigial ATP synthase complex. The complex was purified from mitoplasts derived from wild-type (WT) HAP1 and HAP1-A12 cells and analyzed by SDS/PAGE. (B) ATP synthase and the vestigial complex purified from mitoplasts from 143B ρ+ and ρ0 cells and analyzed by SDS/PAGE. (C and D) Relative abundance of subunits of ATP synthase and two forms of the ATPase inhibitor protein, IF1-M1 and IF1-P (SI Appendix, Fig. S7). The complex and residual complex were purified from a 1:1 mixture of SILAC-labeled wild-type HAP1 cells and HAP1-A12 cells (C) or 143B ρ+ and ρ0 cells (D), and tryptic peptides were analyzed by quantitative mass spectrometry. The experiments were performed twice with reciprocal protein labeling. The bars represent median values of both relative abundance ratios determined for proteins identified in the complementary SILAC-labeling experiments. Error bars show the range of the two values. The proteins in A and B were stained with silver. Subunits were identified by mass spectrometric analysis of tryptic digests of bands from a duplicate gel stained with Coomassie blue dye. The histograms are derived from the data in SI Appendix, Fig. S6, and Datasets S1–S4. Isoforms 1 and 2 of the f-subunit (f-1 and f-2) (SI Appendix, Fig. S7) were identified in human cells.