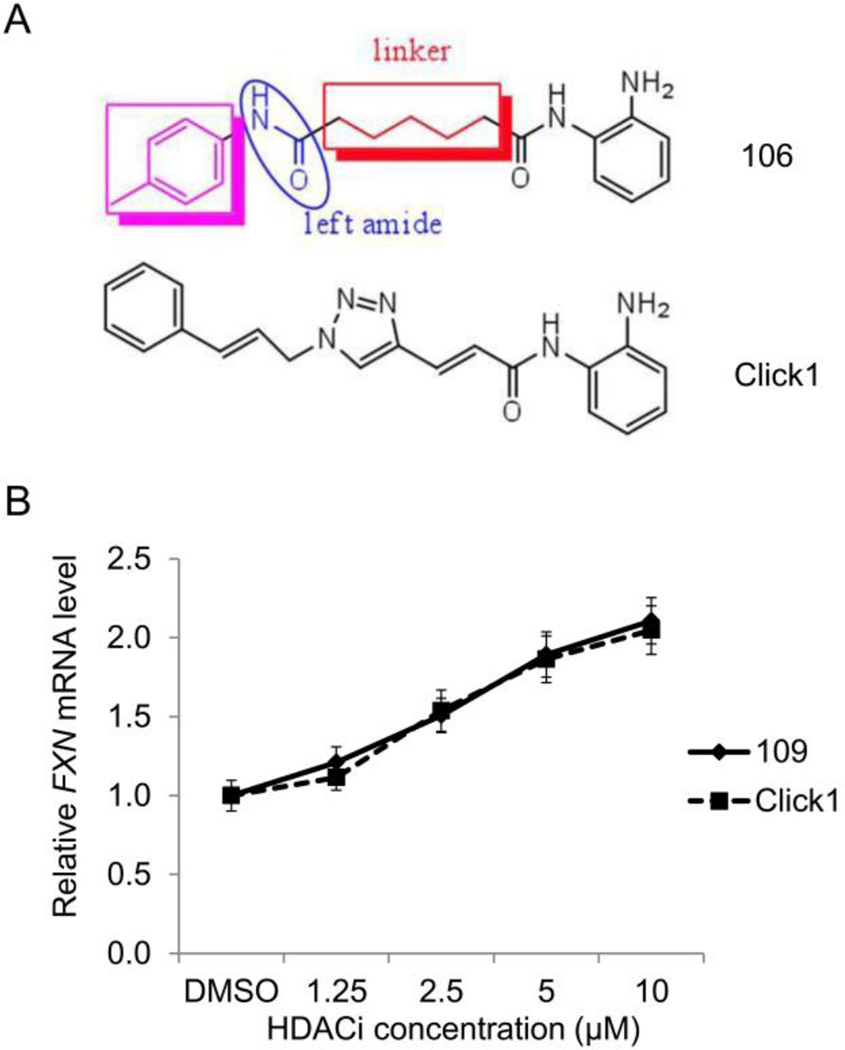
Figure 4. Compounds with improved pharmacological properties.
(A) Structure of 106, highlighting the cap group (purple), left amide, and linker region. Brain penetration can be improved by elimination of the left amide, and replacement with an ether, olefin, or ketone. Metabolic stability can be improved by introducing a non-saturated linkage adjacent to the right amide, which prevents formation of a benzimidazole. Cu(I)-catalyzed click chemistry was used to generate compound click-1, where a triazole is generated in the linker region of the histone deacetylase inhibitor [81]. (B) Efficacy of click-1 in neuronal cells derived from FRDA iPSCs, as measured by qRT-PCR, compared to HDACi 109.