Figure 1. Measurement of aPL tests through pregnancy according to aPL positivity at baseline.
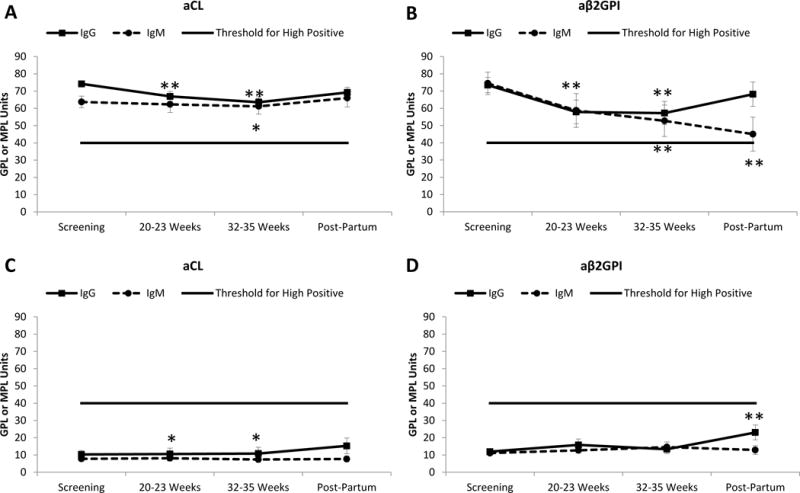
Patients are classified according to positivity for each aPL test at baseline.
A. Variation in aCL titers, among aCL-positive patients at screening (n=90 for IgG and n=29 for IgM). B. Variation in aβ2GPI titers, among aβ2GPI-positive patients at screening (n=59 for IgG and n=26 for IgM). C. Variation in aCL titers, among aCL-negative patients at screening (n=59 for IgG and n=120 for IgM). D. Variation in aβ2GPI titers, among aβ2GPI-negative patients at screening (n=89 for IgG and n=122 for IgM).
aPL positivity was defined for each test. aCL: IgG ≥40 GPL units; IgM ≥40 MPL units; and anti-β2GPI: IgG ≥40 GPL units; IgM ≥40 MPL units. To be considered positive, each test met these criteria at least twice between 6 weeks and 5 years apart, with one determination during the PROMISSE pregnancy at a core lab (4). Negative aCL or aβ2GPI titers were defined as <40 GPL or MPL units.
Values represent mean ± SEM. *p<0.05 **p<0.01 compared to screening using Wilcoxon-Paired test.
Abbreviations: aCL: Anticardiolipin antibodies; aβ2GPI: anti-β2 Glycoprotein I antibodies
