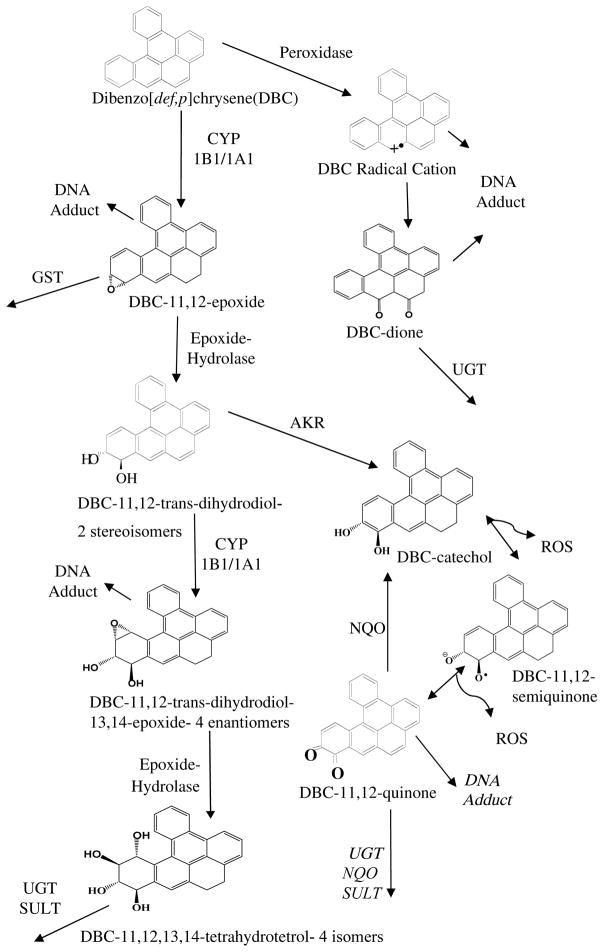
Figure 1. Metabolic Activation of DBC.
Pro-carcinogenic DBC is activated to reactive intermediate metabolites by several enzymatic processes. Peroxidase activation can form a radical DBC species that adduct DNA. The CYP1B1 or CYP1A1 pathways can convert DBC to several metabolites that form DNA adducts, be conjugated for elimination or further metabolized to more reactive intermediates. The primary DBC carcinogenic metabolite is the (−)-anti-trans-11,12-diol-13,14-epoxide. The 11,12-trans-dihydrodiol is additionally a substrate for to aldo-keto reductase (AKR) formation of a catechol which can then undergo redox cycling. As information on the fate of DBC quinones was not available we relied on the prototypical PAH, benzo[a]pyrene (BaP), for which evidence has been published with respect to DNA adduction and further metabolism by UDP-glucuronosyl transferase (UGT), NADPH quinone oxidoreductase (NQO) and sulfatase (SULT)51–54. It is acknowledge that the reactivity and metabolism of DBC quinones may differ from BaP.