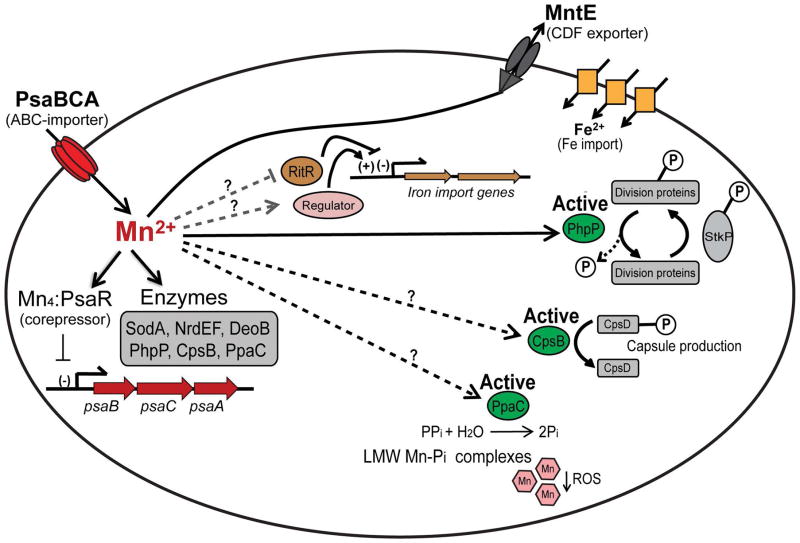
Figure 6. Overview of manganese metabolism in S. pneumoniae.
Mn is imported into the cell by PsaBCA. Imported Mn is used to metallate a relatively small number of Mn-utilizing enzymes, including SodA, NrdEF, DeoB, PpcA, CpsB, and PhpP; see text for functional descriptions. During Mn-replete conditions Mn-bound PsaR represses transcription of psaBCA, thereby reducing Mn import. Excess Mn is effluxed from the cytoplasm by the constitutively expressed MntE exporter (Martin and Giedroc, 2016; Rosch et al., 2009). Failure of MntE to properly export Mn results in accumulation of cell-associated Mn (see Fig. 2) and hyperactivation of Thr/Ser-phosphatase PhpP, which in turn leads to hyper-dephosphorylation of cell division proteins. Excess Mn may also inhibit the orphan response regulator, which leads to induction of expression of Fe import genes. Mn may also activate an, as yet, unidentified regulatory protein that is involved in regulating Fe homeostasis. In addition, Mn may regulate capsule biosynthesis via the Mn-dependent protein phosphatase-kinase pair, CpsB/CpsD, or help reduce reactive oxygen species via formation of low molecular weight (LMW) Mn-phosphate (Pi) complexes through activation of the Mn-dependent inorganic pyrophosphatase, PpaC.