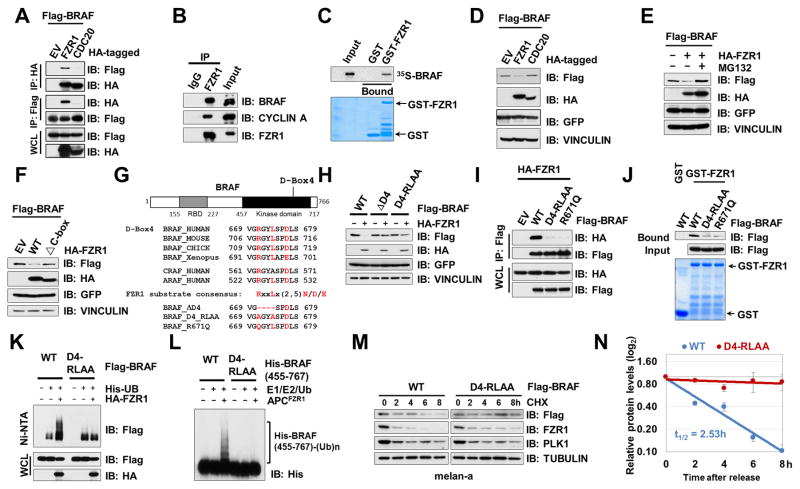
Figure 3. APCFZR1 Promotes BRAF Ubiquitination in a D-box-Dependent Manner.
(A) BRAF specifically bound to FZR1, but not CDC20 in cells. Immunoblot (IB) analysis of whole cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with HA-FZR1 or HA-CDC20 together with the Flag-BRAF construct. 36 hours post-transfection, cells were pretreated with 10 μM MG132 for 10 hours before harvesting.
(B) Endogenous BRAF bound to endogenous FZR1. IB analysis of WCL and anti-FZR1 IP derived from HeLa cells.
(C) In vitro transcribed and translated BRAF (IVT-35S-BRAF) bound to purified recombinant GST-FZR1. Autoradiography of 35S-labelled BRAF bound to bacterially purified GST-FZR1, but not the GST recombinant protein.
(D) FZR1, but not CDC20, promoted the degradation of BRAF. IB analysis of WCL derived from 293 cells transfected with HA-FZR1 or HA-CDC20 with Flag-BRAF constructs. GFP serves as an internal transfection control.
(E) FZR1-mediated BRAF degradation could be blocked by the 26S proteasome inhibitor, MG132. IB analysis of WCL derived from 293 cells transfected with Flag-BRAF and EV or HA-FZR1 constructs. 10 μM MG132 was used to inhibit the 26S proteasome where indicated. GFP serves as an internal transfection control.
(F) APC-binding deficient ΔC-box-FZR1 failed to promote BRAF degradation. IB analysis of WCL derived from 293 cells transfected with Flag-BRAF and HA-tagged WT-FZR1 or E3 ligase activity deficient ΔC-box-FZR1 constructs. GFP serves as an internal transfection control.
(G) Sequence alignments of the putative D-boxes containing region between BRAF proteins from various species as well as a schematic representation of the various D-boxes deletion mutants generated and used in the following studies.
(H) D-box4-deleted or mutated BRAF mutants were resistant to FZR1-mediated degradation. IB analysis of WCL derived from 293 cells transfected with the indicated Flag-BRAF mutants with HA-FZR1 where indicated. GFP serves as an internal transfection control.
(I) D-box4 mutants of BRAF failed to bind FZR1. IB analysis of WCL derived from 293 cells transfected with the indicated Flag-tagged WT- or mutant BRAF constructs with HA-FZR1 where indicated. GFP serves as an internal transfection control.
(J) D-box4 mutated BRAF failed to bind FZR1 in vitro. GST pull down analysis to determine WT-, D4-RLAA, or R671Q mutant form of BRAF bound to the indicated GST fusion proteins.
(K) FZR1 promoted ubiquitination of WT-BRAF, but not D-box4 mutated BRAF, in cells. APCFZR1 promotes BRAF ubiquitination in vivo. IB analysis of WCL and subsequent His-tag pull-down in 6 M guanine-HCl containing buffer derived from 293 cells transfected with the indicated plasmids. Cells were pre-treated with 10 μM MG132 for 10 hours to block the proteasome pathway before harvesting.
(L) APCFZR1 promoted BRAF ubiquitination in vitro. Bacterially purified WT- and D4-RLAA-His-BRAF kinase domain (455–767) proteins were incubated with the APCFZR1 complex purified from G1 phase-arrested HeLa cell extract together with purified E1, E2 and ubiquitin as indicated at 30°C for 60 minutes before being resolved by SDS-PAGE and probed with the anti-His antibody.
(M–N) D-box4 mutated BRAF displayed an extended half-life compared with its WT counterpart. melan-a cells ectopically expressing WT- or D4-RLAA-BRAF were treated with 20 μg/ml cycloheximide (CHX) for the indicated time periods before harvesting. Equal amounts of whole cell lysates (WCL) were immunoblotted with the indicated antibodies (M). (N) Quantification of Flag-BRAF band intensities was plotted as mean ± SD from three independent experiments, Flag-BRAF bands were normalized to TUBULIN, then normalized to the t = 0 time point.