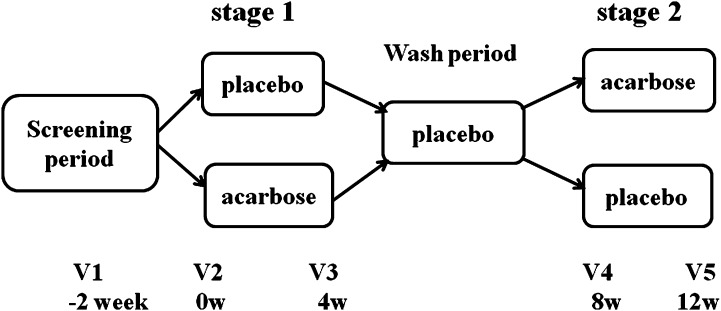
Fig. 1.
Study design. The study consisted of a screening visit, 4 weeks of treatment with acarbose or placebo (stage 1), a 4-week wash period in which all participants received placebo, and then 4 weeks of treatment with the alternative medication (stage 2). Eligible participants (n = 52) were randomly allocated to one of the two treatment sequences