Abstract
Surgery is a crucial intervention and provides a chance of cure for patients with cancer. The perioperative period is characterized by an increased risk for accelerated growth of micrometastatic disease and increased formation of new metastatic foci. The true impact for cancer patients remains unclear. This review summarizes the often fragmentary clinical and experimental evidence supporting the role of surgery and inflammation as potential triggers for disease recurrence. Surgery induces increased shedding of cancer cells into the circulation, suppresses anti-tumor immunity allowing circulating cells to survive, upregulates adhesion molecules in target organs, recruits immune cells capable of entrapping tumor cells and induces changes in the target tissue and in the cancer cells themselves to enhance migration and invasion to establish at the target site. Surgical trauma induces local and systemic inflammatory responses that can also contribute to the accelerated growth of residual and micrometastatic disease. Furthermore, we address the role of perioperative factors including anesthesia, transfusions, hypothermia, and postoperative complications as probable deleterious factors contributing to early recurrence. Through the admittedly limited understanding of these processes, we will attempt to provide suggestions for potential new therapeutic approaches to target the protumorigenic perioperative window and ultimately improve long term oncologic outcomes.
More than a quarter of people worldwide will ultimately be affected by cancer (1) and surgical removal remains a mainstay in the cure and control of most solid cancers. Although surgical excision of primary or even metastatic tumors can save or extend life, it has long been acknowledged that the surgical insult itself may precipitate or accelerate tumor recurrence. The notion that tumor removal may enhance tumor recurrence was cautioned at the turn of the 20th century by Paget and Halsted, who found that patients who underwent resection of their cancer did not survive as long as those managed expectantly (2). Such reports had generally been dismissed as anecdotal until more recent evidence demonstrated that the surgical operation may generate a permissive environment for tumor growth. Several groups have recently revived the idea that addressing the mechanisms involved in the protumorigenic perioperative period may provide insight into ways to improve cancer outcomes (3, 4).
Trauma and inflammation have long been associated with enhanced tumor growth after being first described by Virchow (4). The propensity of circulating tumor cells in experimental animals to metastasize to sites of physical or chemical injury was repeatedly shown by mid-20th century investigators (5). The innate immune system is activated both systemically and locally as a result of tissue trauma precipitating a complex multifaceted inflammatory response. Of course, such inflammation is fundamental to the elimination of potential pathogens and tissue healing, but these local and systemic inflammatory alterations seem to provide fertile soil for both capture of circulating tumor cells and their subsequent growth. It has been demonstrated in animal models that sites of injury are a preferential area for tumor growth and that surgical trauma enhances loco-regional metastases (5). Several experimental trials clearly demonstrate that tumor removal is followed by accelerated tumor growth both locally and at distant sites (4, 6). Moreover, we recently demonstrated that liver metastatic burden is significantly increased after surgical stress where surgery induced both formation of new metastatic foci as well as locoregional acceleration of tumor growth (7).
Despite overwhelming evidence from experimental studies, clinical studies have not been as persuasive, and the concept is still subject to debate and the true impact it has on cancer patients remains unclear. Much reliance has been placed on anecdotal evidence describing the acceleration of growth of peritoneal metastatic deposits after laparotomy (8). In addition, some studies suggested that open oncological resections were associated with shorter disease free survival compared to minimally invasive resections, a concept that is strongly corroborated by experimental data (8). Again, the findings that different operative approaches influence the oncological outcomes is strong evidence that the tissue trauma inflicted during tumor removal can influence the subsequent growth of residual neoplastic disease.
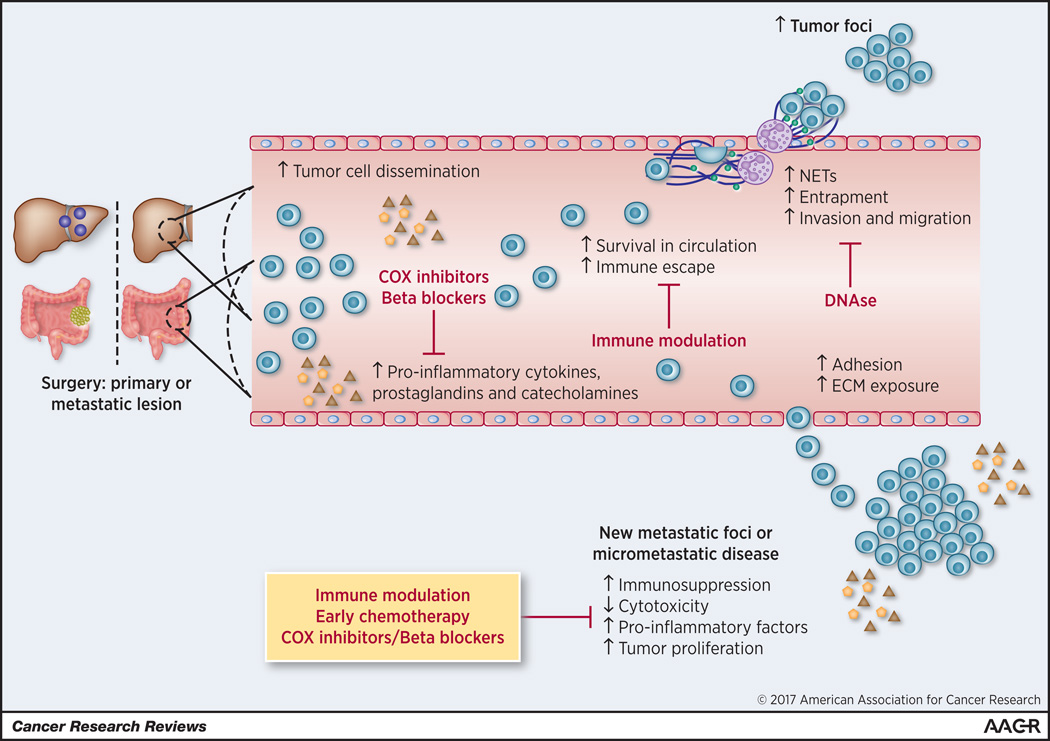
In this review, we will briefly summarize the growing evidence that support the concept that surgery to eliminate the cancer can actually serve to increase the establishment of new metastases and accelerate growth of residual and micrometastatic disease. In addition, we will review the perioperative factors that may enhance postoperative tumor growth and the therapeutic implications that might be useful in counteracting this phenomenon (Figure 1).
Figure 1.
Effects of tumor removal on promotion of metastases. These effects include accelerated growth of micrometastases and establishment of new metastatic foci. Surgery increases tumor cell dissemination, increased circulating tumor cells’ survival by enhancing immune evasion, enhanced entrapment at metastatic site and increased invasion and migration capabilities to establish new metastatic foci. Surgery can also induce changes in the environment of micrometastatic disease to enhance its growth. Multiple therapeutic approaches illustrated in this diagram can be considered to target the protumorigenic inflammatory changes in the perioperative period.
Surgery induces the formation of new metastatic disease
In order for a cancer cell to successfully metastasize to a distant organ, a complex cascade of events need to occur (9). A cancer cell must reach the circulation, survive the host defensive mechanisms, get entrapped at a regional or distant site, and finally invade and prosper within the new metastatic site. Patients with a primary cancer routinely have circulating tumor cells. But metastasis in general is an inefficient process and the majority of cancer cells reaching the circulation are quickly destroyed (9). However, all tissue trauma, including the sterile dissection carried out by surgeons, elicits a cascade of local and systemic cellular and humoral inflammation which has the potential to capture the cancer cell and support its survival and metastatic growth.
The unavoidable damage to the patients’ tissues during excision and manipulation of the tumor being resected and its vasculature have been shown to result in shedding of tumor cells into the blood and lymphatic circulation (10). Handling of the tumor can result in at least a tenfold rise in circulating tumor cells (11). Furthermore, the level of circulating cancer cells before and during surgery have been shown to be a strong predictor of recurrence (12). In addition to the dissemination of circulating cells, several postoperative changes help the cancer cells survive in the circulation and increase the likelihood for distant implantation. Macrophages and natural killer (NK) cells play a critical role in the elimination of circulating cancer cells and the prevention of metastases formation (13, 14). In experimental models, the increase in tumor growth after surgery was accompanied by diminished NK cytotoxicity and impairment of macrophage function which was proportional to the extent and magnitude of surgery (13, 14).
Additionally, a number of studies support the hypothesis that the acute inflammatory response to surgery favors the capture of tumor cells in foreign locations. For example, pro-inflammatory cytokines such as IL-1 and TNF-α can stimulate the adhesion of the viable circulating cancer cells (6). Surgery induces changes in mesothelial cells in the peritoneal cavity that causes them to retract and detach thereby exposing the underlying extracellular matrix with which cancer cell can interact (15). Indeed, inhibiting the tumor cell-ECM interactions by blocking α2 integrins significantly decreased the surgical induced acceleration of liver metastases in mice (16).
The neutrophil influx that follows surgical trauma seems to further promote tumor capture and growth (7). Neutrophils react to injured tissue by forming neutrophil extracellular traps (NETs) consisting of extracellular extrusion of web-like DNA that can ensnare circulating tumor cells. In addition to their mechanical function, the DNA strands are studded with a variety of proinflammatory molecules which are crucial to the capture of tumor cells and augmented growth of metastases in surgically manipulated livers (7). The inhibition of NETs after surgery powerfully inhibit the previously observed accelerated development of new metastatic disease. In humans undergoing resection of hepatic colorectal metastases, the greater the serum evidence of NET formation the higher the risk of recurrence (7). Thus both experimental and clinical evidence provides support for the idea that the environment generated after tumor removal can affect long-term cancer-related outcomes.
The liver is peculiarly susceptible to metastases from primary gastrointestinal solid tumors. Among the many potential reasons is that surgical trauma can impair the integrity of liver endothelial cells with reduced expression of tight junction proteins to facilitate cancer cell migration into the liver parenchyma (6). In addition, the catecholamine and prostaglandins released and the NETs formed in response to the surgical trauma can promote the metastatic potential of the adhered circulating cancer cells by increasing tumor cell migration and invasion into the distant organ (3, 7). Surgical trauma thus synchronizes the increased numbers of circulating cancer cells, the suppressed anti-tumor immunity, and the pro-metastatic environment of the targeted organs within the hepatic gastrointestinal watershed.
Surgery promotes the growth of micrometastatic and residual disease
Metastatic cancer cells may leave the primary tumor early during its development and form clinically undetectable micrometastases at distant sites. These islands of clinically undetectable micrometastases can remain in a dormant equilibrium between cellular proliferation and apoptosis (17). The local and systemic inflammatory events associated with surgical trauma can unpredictably unleash their potential for growth (17). In addition to the soluble factors which facilitate distant tumor growth after surgery, the removal of the primary tumor itself can release the inhibitory control exerted by primary tumors which act keep the growth of dormant metastases in check. This ability of the primary tumor to retard the growth of metastatic foci is known as concomitant tumor resistance and the topic has been reviewed comprehensively by Ruggiero (18). Primary tumors secrete both proangiogenic factors and inhibitors of angiogenesis. In the microenvironment of the primary tumor, the inducers overcome the effects of the inhibitors because the new vessels essential for progressive tumor growth are present. However, when shed into the circulation, levels of the more labile inducers fall off rapidly, whereas levels of the more stable inhibitors create a systemic antiangiogenic environment that prevents small distant micrometastases from inducing neovascularization and growing. As a result, these micrometastases remain small and dormant. Upon removal of the primary tumor, inhibitor levels fall and the previously dormant metastases expand with renewed vigor. Thus, tumor extirpation can result in turning on the angiogenic switch resulting in decrease in the systemic levels of antiangiogenic factors such as angiostatin, endostatin and thrombospondin (3). Thus the reduced expression of antiangiogenic factors, added to the surgery-induced increases in the levels of growth factors and of proangiogenic compounds, might enable undetectable dormant micrometastatic disease to undergo the angiogenic switch and quickly grow (3, 7, 18).
Surgery may also prompt immune escape by triggering postoperative downregulation of the adaptive immune response. For example, the overall level of circulating dendritic cells (DC), essential for immune surveillance, decrease following tumor removal. Experimental data have shown that supplementing tumor bearing mice with dendritic cell vaccine significantly attenuates the effect of surgery on the growth of existing tumor (8). Moreover, surgery induces impaired T helper 1 (Th1) functions in humans (19). Impairment of Th1 responses, normally an essential step in specific cellular immunity and proliferation of cytotoxic T cells, might hamper antitumor cytotoxicity as well. Surgery induced immunosuppression persists for weeks and is longer after laparotomy compared to laparoscopy (20). Furthermore, surgery induces neutrophil recruitment and NET formation at the site of injury that can persist for weeks and induce growth of residual disease by activating Stat3 and NFκB pathways (7). Thus the perioperative period may represent an immunological gap during which the extracellular milieu is more permissive to residual tumor growth.
Perioperative factors affecting cancer recurrence
In addition to the previously mentioned changes directly related to surgical treatment, there are countless perioperative variables that can alter the oncological outcomes. These include anesthetic management, blood transfusion, hypothermia, and the evolution of postoperative complications. Experimental data have shown that anesthetic agents can directly influence the tumor micro-environment and growth (3). Similarly, the use of opioids to control pain have been shown in animals and humans to activate stress responses, suppress cell mediated immunity, increase angiogenesis, and promote the progression of metastatic disease (3). Evidence from clinical observational studies suggests that both general anesthesia and opioid analgesics increase recurrence rates (3) and if confirmed by more rigorous trials, might encourage changes in anesthetic and pain management.
Blood transfusions are often required in the perioperative period. It has been repeatedly shown that transfusion is independently associated with a significant increase in mortality in several types of cancer (3). Transfusion of blood products can cause immunosuppression, increase in prostaglandin production and suppression of NK cell activity (3). These negative effects are magnified when more units are transfused, the use of whole blood rather than packed red blood cells, and with the transfusion of units subjected to longer storage (3).
Despite efforts to maintain body temperature during prolonged operations, systemic hypothermia is commonly encountered and even a few degrees of perioperative hypothermia can have immunosuppressive consequences (21). Hypothermia can also cause abnormalities in the platelet function and in the coagulation cascade and thus may potentially increase the requirements for blood transfusion (22). In rodent models, hypothermia causes significant increase in tumor growth and is also associated with suppressed NK function and increased susceptibility to developing new metastatic disease.
Postsurgical infections in patients with cancer have been associated with adverse oncologic outcomes independent of the morbidity associated with the infectious insult (23). This phenomenon has been observed across a broad range of malignancies, including lung, esophageal, breast, ovarian, and colorectal cancer; severe postoperative infectious complications, are significantly associated with an increased rate of death from metastatic disease (24). In mice models, sepsis is a strong stimulus for formation of NETs that promote early adhesion of tumor cells to distant organ sites and facilitate metastatic disease progression (25). Furthermore, invasive postoperative infections and translocation of bacteria from the gastrointestinal tract into systemic circulation can reduce cancer cell apoptosis and enhance resistance to chemotherapeutic agents (8). LPS is also proangiogenic and a potent proinflammatory mediator that could contribute to tumor growth (26).
Perioperative Therapeutic Options
There is a wealth of clinical and experimental data supporting the concept that tumor growth may accelerate in the immediate perioperative period, potentially offering a window of opportunity in which to alter oncological outcomes. The administration of chemotherapy immediately postoperatively has been previously studied. A single short dose of cyclophosphamide or anthracycline-based agents administered during the postoperative period significantly enhanced long term survival in breast cancer (8, 27). However, immediate postoperative chemotherapy has been virtually abandoned for fear of its adverse impact on infection control and wound healing. In addition, similar to surgery-induced tumor progression, chemotherapy and other cancer-directed treatments themselves can induce a cascade of host events to support tumor growth and spread. The above issue has been comprehensively reviewed by Ebos et al (28).
Aside from perioperative chemotherapy, little study has been devoted to favorably altering the subsequent course of occult metastatic cancer in the perioperative period. Neuroendocrine mediators are significantly elevated as a response to surgery and can directly stimulate prometastatic capacities of cancer cells and suppress cell mediated immunity (3). Reversing the neuroendocrine responses to surgical trauma has promise. Blocking the rise of catecholamines and prostaglandins in the perioperative period using betablockers or COX inhibitors may prove beneficial. There are a few randomized controlled trials and retrospective cohort studies that have studied the impact of perioperative treatment with COX inhibitors or beta blockers but the results are inconclusive (3, 29).
Several immunomodulatory approaches performed in animals and/or humans have shown promise to ameliorate the surgery-induced immunosuppression and restore antitumor cytotoxicity in the perioperative period. These include administration of interferon γ, IL-2, granulocyte macrophage-colony stimulating factor (GM-CSF), and the transfer of interleukin 1-generated lymphokine-activated killer cells (6). Tumor vaccine such as dendritic-cell vaccines are also currently being investigates as a potential strategy during this period (30). By providing an adequate adaptive immunity against the circulating tumors and micrometastatic disease, these strategies might overcome surgery-induced immunosuppression and potentially improve outcomes. Another promising approach derives from experimental studies that show that blocking the innate immune response notably neutrophils from forming NETs by administering DNAse can decrease metastases formation, presumably by decreasing entrapment of circulating cancer cells at metastatic sites (7, 25). This is supported by human data showing that increased NET formation in the immediate postoperative period is associated with a significant increase in cancer recurrence (7). Thus, the use of DNAse to inhibit NETs is another promising approach for potential clinical application perioperatively and clinical trials are warranted.
The local and systemic inflammatory response to tissue injury seems to underlie many aspects of the protumorigenic outcome for potentially curative surgical resections. Inflammation reflects a coordinated response of chemokines, cytokines, and inflammatory cells which has received much study. Less studied is the resolution of inflammation which is an active equally complex process. Specific mediators of importance in the resolution of inflammation have recently been discovered, and could conceivably prove useful during the perioperative period in the relative absence of microbial pathogens (31). Among the mediators are specialized lipid molecules such as lipoxins, resolvins, protectins and maresins; proteins and peptides in the annexin A1 family; prostaglandin E2 and activators of the Peroxisome proliferator activated receptor (PPAR) family of nuclear hormone receptors (31). Together, with independent and overlapping mechanisms, these pro-resolution mediators act to downregulate proinflammatory agents derived from platelets, neutrophils and macrophages leading to a phenotypic switch toward return to a homeostatic normalcy. Pharmacological manipulation of these pro-resolution mechanisms may well prove useful in the reverse the pro-metastatic tendencies in the perioperative period.
As for modulating perioperative clinical factors, in view of the available experimental and clinical evidence detailed above, it may be more advantageous to use regional anesthesia and non-opioid analgesics when performing oncologic resections. Similarly, the reduction of blood transfusions, avoiding whole blood transfusions, using units with shorter shelf life, and maintaining normothermia during surgery and the immediate postoperative period may prevent the associated immunosuppression that may adversely affect oncologic outcomes. Interestingly, incorporating the increasingly implemented Enhanced Recovery after Surgery (ERAS) pathways when feasible may provide oncologic benefits as many of the guidelines of ERAS overlap with the principles mentioned above (32). ERAS pathways have also been shown to significantly decrease postoperative complications and thus has the potential, although remains unstudied, to improve long term oncologic outcomes.
Concluding remarks
Metastasis is a common cause of morbidity and mortality in cancer patients. Both experimental and clinical evidence lend support to the idea that surgery which is intended to be a curative option to remove and reduce tumor mass, can paradoxically also augment development of metastases. If one can address those factors in the peri-operative period which act to foster capture and promotion of metastases, the immediate postoperative period might become a unique window to control residual malignant cells.
Acknowledgments
Source of Funding: ST was supported by the National Institutes of Health (T32CA113263). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. 2015. CA: a cancer journal for clinicians. 2015 Jan-Feb;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Halsted WS. I. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Annals of surgery. 1907 Jul;46(1):1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nature reviews Clinical oncology. 2015 Apr;12(4):213–226. doi: 10.1038/nrclinonc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008 Nov;19(11):1821–1828. doi: 10.1093/annonc/mdn386. [DOI] [PubMed] [Google Scholar]
- 5.Murthy SM, Goldschmidt RA, Rao LN, Ammirati M, Buchmann T, Scanlon EF. The influence of surgical trauma on experimental metastasis. Cancer. 1989 Nov 15;64(10):2035–2044. doi: 10.1002/1097-0142(19891115)64:10<2035::aid-cncr2820641012>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Annals of surgery. 2009 May;249(5):727–734. doi: 10.1097/SLA.0b013e3181a3ddbd. [DOI] [PubMed] [Google Scholar]
- 7.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Lougharn P, Mowen K, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer research. 2016 Jan 12; doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffey JC, Smith MJ, Wang JH, Bouchier-Hayes D, Cotter TG, Redmond HP. Cancer surgery: risks and opportunities. BioEssays : news and reviews in molecular, cellular and developmental biology. 2006 Apr;28(4):433–437. doi: 10.1002/bies.20381. [DOI] [PubMed] [Google Scholar]
- 9.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature reviews Cancer. 2002 Aug;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Annals of surgery. 2000 Jul;232(1):58–65. doi: 10.1097/00000658-200007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer research. 1974 May;34(5):997–1004. [PubMed] [Google Scholar]
- 12.Koch M, Kienle P, Hinz U, Antolovic D, Schmidt J, Herfarth C, et al. Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Annals of surgery. 2005 Feb;241(2):199–205. doi: 10.1097/01.sla.0000151795.15068.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rushfeldt C, Sveinbjornsson B, Seljelid R, Smedsrod B. Early events of hepatic metastasis formation in mice: role of Kupffer and NK-cells in natural and interferon-gamma-stimulated defense. The Journal of surgical research. 1999 Apr;82(2):209–215. doi: 10.1006/jsre.1998.5532. [DOI] [PubMed] [Google Scholar]
- 14.Oosterling SJ, van der Bij GJ, Meijer GA, Tuk CW, van Garderen E, van Rooijen N, et al. Macrophages direct tumour histology and clinical outcome in a colon cancer model. The Journal of pathology. 2005 Oct;207(2):147–155. doi: 10.1002/path.1830. [DOI] [PubMed] [Google Scholar]
- 15.Oosterling SJ, van der Bij GJ, Bogels M, ten Raa S, Post JA, Meijer GA, et al. Anti-beta1 integrin antibody reduces surgery-induced adhesion of colon carcinoma cells to traumatized peritoneal surfaces. Annals of surgery. 2008 Jan;247(1):85–94. doi: 10.1097/SLA.0b013e3181588583. [DOI] [PubMed] [Google Scholar]
- 16.van der Bij GJ, Oosterling SJ, Bogels M, Bhoelan F, Fluitsma DM, Beelen RH, et al. Blocking alpha2 integrins on rat CC531s colon carcinoma cells prevents operation-induced augmentation of liver metastases outgrowth. Hepatology. 2008 Feb;47(2):532–543. doi: 10.1002/hep.22013. [DOI] [PubMed] [Google Scholar]
- 17.Michelson S, Leith JT. Dormancy, regression, and recurrence: towards a unifying theory of tumor growth control. Journal of theoretical biology. 1994 Aug 21;169(4):327–338. doi: 10.1006/jtbi.1994.1155. [DOI] [PubMed] [Google Scholar]
- 18.Chiarella P, Bruzzo J, Meiss RP, Ruggiero RA. Concomitant tumor resistance. Cancer letters. 2012 Nov 28;324(2):133–141. doi: 10.1016/j.canlet.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Berguer R, Bravo N, Bowyer M, Egan C, Knolmayer T, Ferrick D. Major surgery suppresses maximal production of helper T-cell type 1 cytokines without potentiating the release of helper T-cell type 2 cytokines. Archives of surgery. 1999 May;134(5):540–544. doi: 10.1001/archsurg.134.5.540. [DOI] [PubMed] [Google Scholar]
- 20.Da Costa ML, Redmond P, Bouchier-Hayes DJ. The effect of laparotomy and laparoscopy on the establishment of spontaneous tumor metastases. Surgery. 1998 Sep;124(3):516–525. doi: 10.1067/msy.1998.89410. [DOI] [PubMed] [Google Scholar]
- 21.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. The New England journal of medicine. 1996 May 9;334(19):1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 22.Frank SM, Higgins MS, Breslow MJ, Fleisher LA, Gorman RB, Sitzmann JV, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology. 1995 Jan;82(1):83–93. doi: 10.1097/00000542-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsuka T, Kitajima Y, Takahashi T, Sato S, Miyoshi A, Kohya N, et al. Infectious complications after gastric cancer surgery accelerate a rapid hepatic recurrence. Hepato-gastroenterology. 2009 Sep-Oct;56(94–95):1277–1280. [PubMed] [Google Scholar]
- 24.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009 Jul;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 25.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. The Journal of clinical investigation. 2013 Jul 1; doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattsby-Baltzer I, Jakobsson A, Sorbo J, Norrby K. Endotoxin is angiogenic. International journal of experimental pathology. 1994 Jun;75(3):191–196. [PMC free article] [PubMed] [Google Scholar]
- 27.van der Hage JA, van de Velde CJ, Julien JP, Floiras JL, Delozier T, Vandervelden C, et al. Improved survival after one course of perioperative chemotherapy in early breast cancer patients. long-term results from the European Organization for Research and Treatment of Cancer (EORTC) Trial 10854. European journal of cancer. 2001 Nov;37(17):2184–2193. doi: 10.1016/s0959-8049(01)00294-5. [DOI] [PubMed] [Google Scholar]
- 28.Ebos JM. Prodding the Beast: Assessing the Impact of Treatment-Induced Metastasis. Cancer research. 2015 Sep 1;75(17):3427–3435. doi: 10.1158/0008-5472.CAN-15-0308. [DOI] [PubMed] [Google Scholar]
- 29.Neeman E, Zmora O, Ben-Eliyahu S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 Sep 15;18(18):4895–4902. doi: 10.1158/1078-0432.CCR-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosolits S, Ullenhag G, Mellstedt H. Therapeutic vaccination in patients with gastrointestinal malignancies. A review of immunological and clinical results. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005 Jun;16(6):847–862. doi: 10.1093/annonc/mdi192. [DOI] [PubMed] [Google Scholar]
- 31.Headland SE, Norling LV. The resolution of inflammation: Principles and challenges. Seminars in immunology. 2015 May;27(3):149–160. doi: 10.1016/j.smim.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Varadhan KK, Lobo DN, Ljungqvist O. Enhanced recovery after surgery: the future of improving surgical care. Critical care clinics. 2010 Jul;26(3):527–547. doi: 10.1016/j.ccc.2010.04.003. x. [DOI] [PubMed] [Google Scholar]