Figure 4. Loss of CK1ε in SCN astrocytes lengthens circadian period in vivo and in vitro.
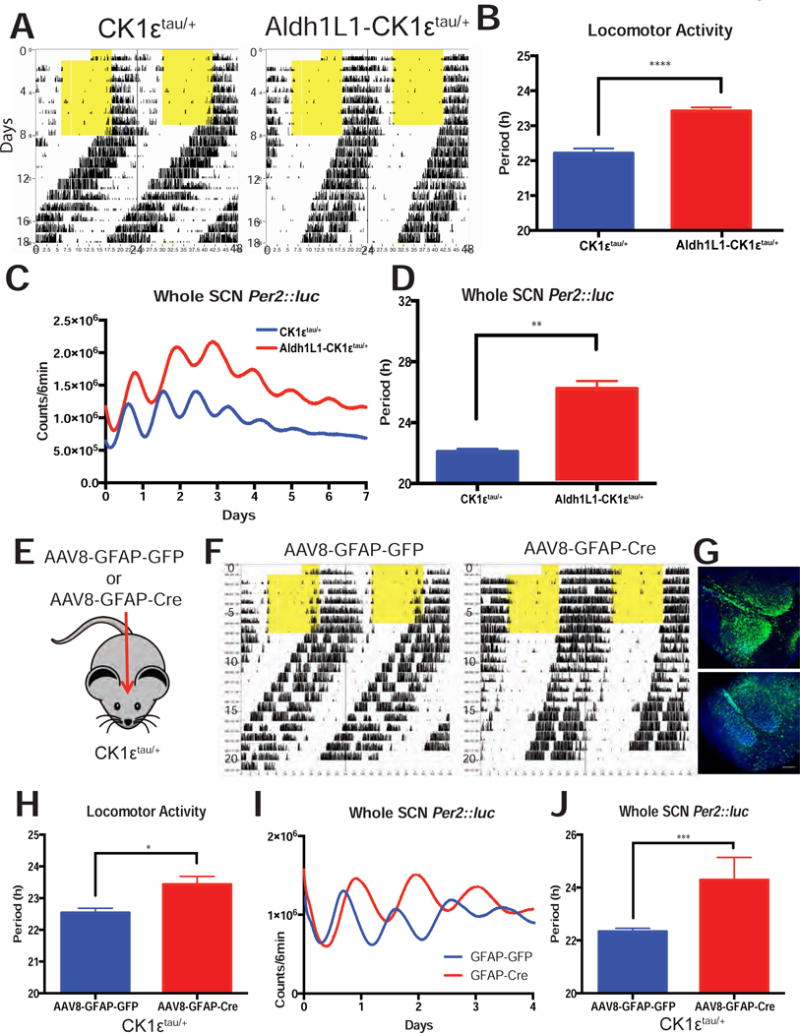
(A) Representative actograms of Aldh1L1- CK1εtau/+ and CK1εtau/+ littermates showing how locomotor activity free-ran in constant darkness with (B) a longer circadian period in mice where the CK1εtau/+ mutation was removed from astrocytes (p < 0.0001, t-test). (C) Representative PER2::luc recordings from the isolated SCN of Aldh1L1- CK1εtau/+ and CK1εtau/+ littermates with (d) a longer circadian period in SCN where the CK1εtau/+ mutation was removed from astrocytes (p < 0.01, t-test). (E) Schematic of a second method to change circadian timing in astrocytes using GFAP AAV injection to CK1εtau/+ SCN in vivo. (F) Representative actograms of two CK1εtau/+ mice injected with either AAV8-GFAP-GFP or AAV8-GFAP-Cre-GFP with (G) successful viral targeting to the SCN. (H) The circadian period of locomotor activity of AAV8-GFAP-Cre injected CK1εtau/+ mice was approximately 1 h longer than controls (p < 0.05, t-test). (I) Representative whole SCN PER2::luc recording from CK1εtau/+ animals injected with either AAV8-GFAP-GFP or AAV8-GFAP-Cre-GFP. (J) Period of PER2::luc recordings from AAV8-GFAP-Cre; CK1εtau/+ SCN was approximately 2 h longer than controls (p < 0.001, t-test). Error bars: mean ± sem.
