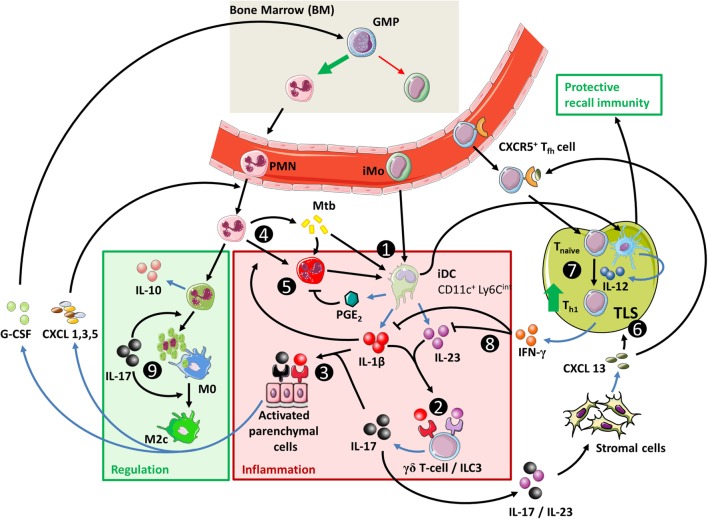
Figure 4.
The IL-23/IL-17 axis in acute tuberculosis. (1) When inflammatory dendritic cells (iDC) recognize Mtb through membrane-bound toll-like receptors, they can secrete IL-1β, IL-23, and prostaglandin E2 (PGE2) (see Figure 1). This occurs more efficiently if iDC are activated through contact with Mtb-infected PMN, which also stimulates their migratory capacity to tertiary lymphoid structures (TLS) and promotes recall immunity (202–206). (2) The combination of IL-1β and IL-23 induces IL-17 production by γδ T-cells and possibly ILC3 (27, 117). (3) Activation of parenchymal cells by IL-17 in combination with IL-1β or other inflammatory mediators ultimately results in PMN influx. (4) PMN contribute to inflammation when stimulated by extracellular Mtb or inflammatory cytokines. (5) Activated PMN readily cause tissue damage through production of ROS and proteases; this effect is suppressed by activated iDC in a PGE2-dependent way (112, 207, 208). (6) IL-23 and IL-17 stimulate the local production of CXCL13 by stromal cells (194, 199). This promotes TLS formation and follicular helper T-cell migration to the site of infection. (7) CD40 ligation in the interaction between (i)DC and CD4+ T-cells is a strong stimulus for IL-12 production over IL-23 (Box 3) and leads to Th1 formation and IFN-γ production. (8) IFN-γ inhibits IL-1β production and shifts IL-23 production to IL-12, thus inhibiting IL-17 production and reinforcing the Th1 response (209). (9) In the absence of inflammatory stimuli, PMN can produce IL-10 and undergo apoptosis. Phagocytosis of apoptotic PMN induces an IL-10-producing regulatory M2c phenotype in macrophages and further contributes to resolution of inflammation.