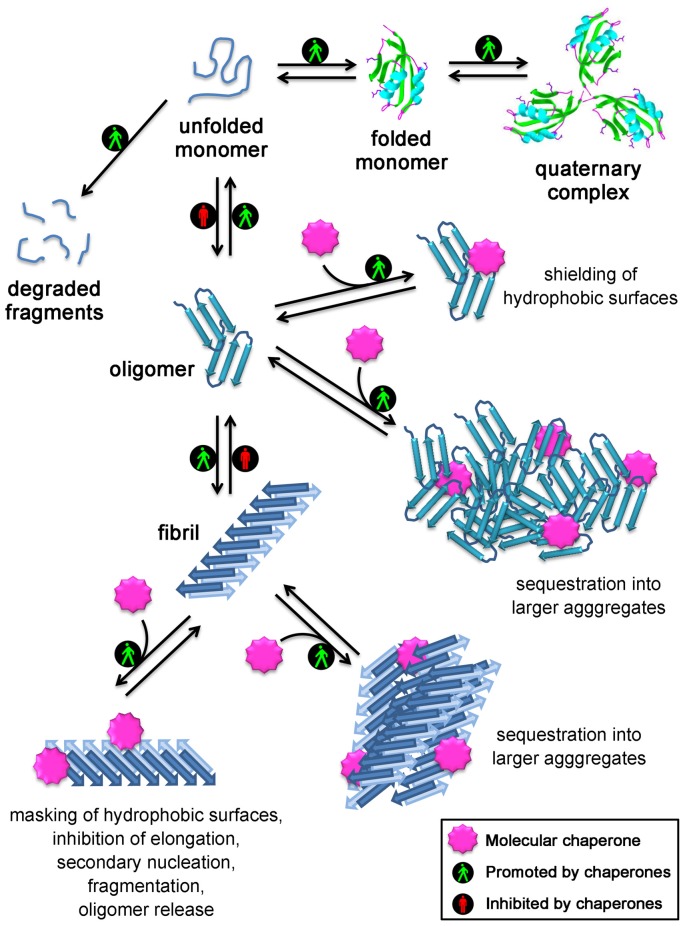
Figure 1.
Mechanisms used by molecular chaperones to maintain proteins in their soluble states and reduce the toxicity of protein aggregates. Chaperones assist protein folding, promote formation and maintenance of multisubunit complexes, mediate protein degradation, inhibit protein aggregation and promote disassembly of undesired protein aggregates. In addition, several strategies are employed by molecular chaperones to reduce the toxicity of protein aggregates: they act on small soluble oligomers by shielding their hydrophobic patches or by sequestering them into larger aggregates; they also promote the clustering of the fibrils, inhibit their elongation, the generation of oligomers through secondary nucleation occurring on the fibril surface, their fragmentation/oligomer release, and mask the reactive hydrophobic residues exposed on the fibril surface.