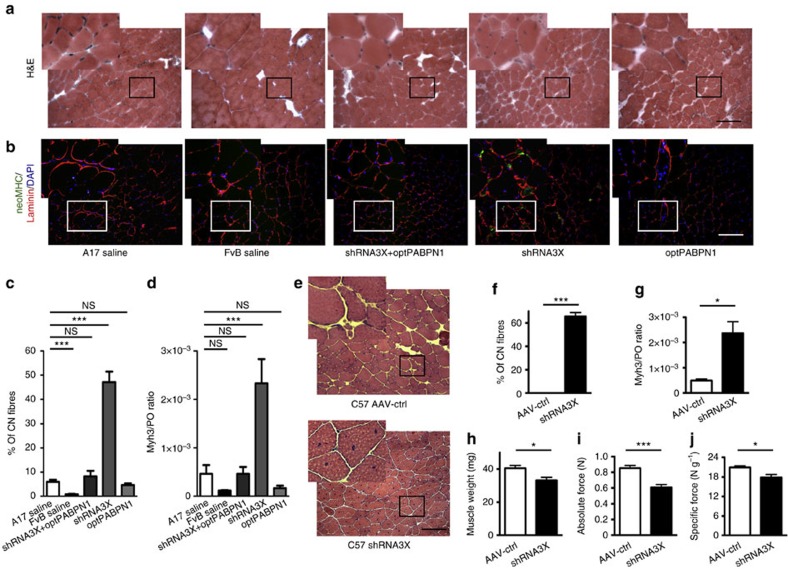
Figure 3. In vivo PABPN1 depletion induces muscle degeneration that can be prevented by co-optPABPN1 expression.
(a) H&E staining of sections from treated muscles of 30-week-old mice indicates that depleting endogenous PABPN1 in A17 muscles increases the amount of centrally nucleated fibres. Bar, 200 μm. (b) Immunostaining for neonatal MHC (green), laminin (red) and DAPI (blue) shows small regenerating neonatal MHC-positive myofibres in muscles treated with shRNA3X only. Bar, 200 μm. (c) Quantification of centrally nucleated fibres indicates that co-injecting AAV-optPABPN1 preserves the amount of fibres with central nuclei to the same level observed in saline-injected A17 muscles, thus preventing muscle degeneration. (d) qRT-PCR analysis of Myh3 mRNA encoding embryonic MHC normalized to the housekeeping gene RplP0 mRNA confirms the regeneration process in shRNA3X-treated muscles. (e) H&E staining of sections from treated muscles shows that depleting endogenous PABPN1 in WT muscles increases the amount of centrally nucleated fibres. Bar, 200 μm. (f) Quantification of centrally nucleated fibres in treated muscles indicates that PABPN1 depletion in WT muscles induce muscle degeneration, as for A17 treated muscles. (g) qRT-PCR analysis of Myh3 mRNA encoding embryonic MHC normalized to the housekeeping gene RplP0 mRNA shows that a regeneration process is ongoing in shRNA3X treated muscles. (h) PABPN1 depletion in WT muscles reduces muscle weight compared to contralateral muscles. (i,j) PABPN1 inhibition decreases both absolute maximal tetanic and specific maximal force generated by TA muscles of wild-type mice. Data are presented as mean±s.e.m. For c,d, one-way ANOVA test with Bonferroni post-hoc test, n=6 (saline-treated A17 or FvB muscles) or n=8 (all the other groups). For f-j, two-tailed Student t-test, n=6, *P<0.05, ***P<0.001, NS, not significant.