Fig. 2.
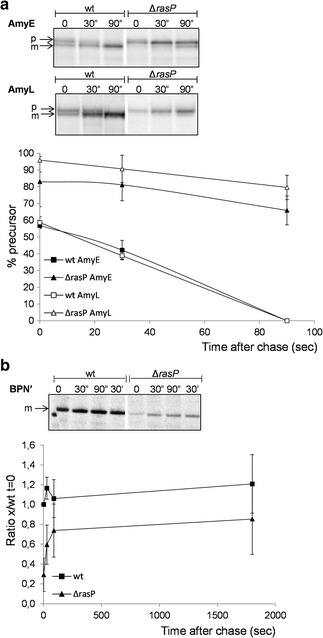
Reduced rates of AmyE, AmyL and BPNʹ secretion in rasP mutant cells. a Processing of the precursor proteins of AmyE or AmyL by signal peptidase was analyzed by pulse-chase protein labeling with [35S]-methionine, immunoprecipitation of AmyE or AmyL from culture samples with specific antibodies, LDS-PAGE and phosphorimaging as described in “Methods”. The positions of precursor (p) and mature (m) forms of AmyE and AmyL are indicated. Data from three independent experiments were analyzed with ImageJ to assess the kinetics of precursor processing, and the results are plotted below the autoradiographs. The plot shows the relative amounts (%) of the precursor forms of AmyE (black symbols) or AmyL (white symbols) in the ΔrasP (triangle) or wt (square) strains at different time points after the chase with non-radioactive methionine (t = 0). Error bars show the standard deviation. b Secretion of BPNʹ was analyzed by pulse-chase labeling with [35S]-methionine, immunoprecipitation from growth medium fractions devoid of cells with specific antibodies, LDS-PAGE and phosphorimaging as described in “Methods”. The position of mature BPNʹ is indicated (m). Data from three independent experiments were analyzed with ImageJ to determine the kinetics BPN’ appearance in the growth medium, and the results are plotted below the autoradiographs. The plot shows the average of the calculated ratio of secreted BPNʹ in the ΔrasP (triangle) or wt (square) strains relative to the amount of BPNʹ secreted immediately after the chase with non-radioactive methionine (t = 0) in the wt strain. Error bars indicate the standard deviation
