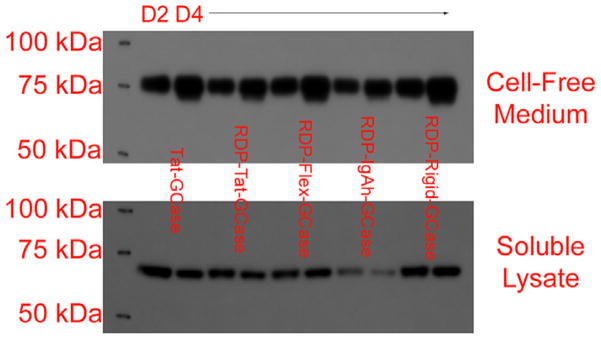
Fig. 1.
The IgK signal peptide facilitated efficient GCase secretion for most engineered enzymes. Predicted molecular weights (polypeptide only): Tat-GCase: 66.1 kDa, RDP-Tat-GCase: 70.7 kDa, RDP-Flex-GCase: 69.7 kDa, RDP-IgAh-GCase: 71.1 kDa, and RDP-Rigid-GCase: 71.2 kDa. Following transient transfection, cell-free medium and HEK 293F soluble lysate were run on 4–12% NuPage Bis-Tris gels and a western blot was performed for the myc epitope tag. For each condition, samples collected both two (D2) and four (D4) days after transfection were run. Three-fold more cell-free supernatant was run on the gels compared to soluble lysate to account for the at least 3× more concentrated soluble lysate. The results shown for the five enzymes were typical for all the secreted glucocerebrosidases. Extending batch duration increased the quantity of secreted protein in the extracellular medium but did not increase the quantity of protein in the soluble lysate. The slightly larger size of the intracellular proteins was predicted to be due to both cleavage of the GCase signal peptide and differences in glycosylation.