Abstract
The aim of this systematic review was taken to investigate the efficacy and safety of docetaxel plus thalidomide vs. docetaxel alone for treating androgen-independent prostate cancer (AIPC). Data were collected from different databases independently by three researchers according to the pre-defined inclusion and exclusion criteria. Total three studies were finally included, indicating that docetaxel plus thalidomide exhibited better survival prognosis and greater prostate-specific antigen (PSA) decline than docetaxel alone. There were no significant differences of hematologic toxicities in two regimens, while the frequency of non-hematologic toxicities was higher in patients with docetaxel plus thalidomide. Briefly, the available evidence indicates potential survival advantage in docetaxel plus thalidomide over docetaxel alone.
Prostate cancer is the most common malignancy in men all over the world1, and the cornerstone of therapy for metastatic prostate cancer so far is androgen deprivation2,3, including hormonal therapies and orchiectomy. However, androgen-independence prostate cancer (AIPC) is still in a therapeutic dilemma since extremely limited treatment options have been successful in prolongation of survival.
Chemotherapy may lead to pain control and improve quality of life, but no benefit in overall survival (OS)4,5. The use of docetaxel, a semisynthetic taxane, has shown notable objective responses and PSA decline6,7,8,9,10,and the later represents the disease improvement11,12,13. In addition, docetaxel has also shown encouraging results in combination with novel agents, such as thalidomide6,7. Thalidomide is a synthetic glutamic acid derivative that was used for pregnancy-associated morning sickness14, but was withdrawn from market for its teratogenic effect of causing dysmelia (stunted limb growth) in humanbeing. Nevertheless, thalidomide was recently proved to have anti-inflammatory, immunomodulatory and antiangiogenic activity15. Thalidomide is now tentatively used in some cancers, including prostate cancer6,7,16. Dohut et al6 and Figg et al7 found that the addition of thalidomide to docetaxel contributed to a larger benefit of survival and a greater PSA decline than docetaxel-alone in AIPC patients.
To date, no systematic review has been designed to observed the efficacy and safety of docetaxel plus thalidomide vs. docetaxel alone for handling AIPC. Consequently, we performed a systematic review to gain an updated perspective on docetaxel plus thalidomide for treating AIPC.
Results
Study selection
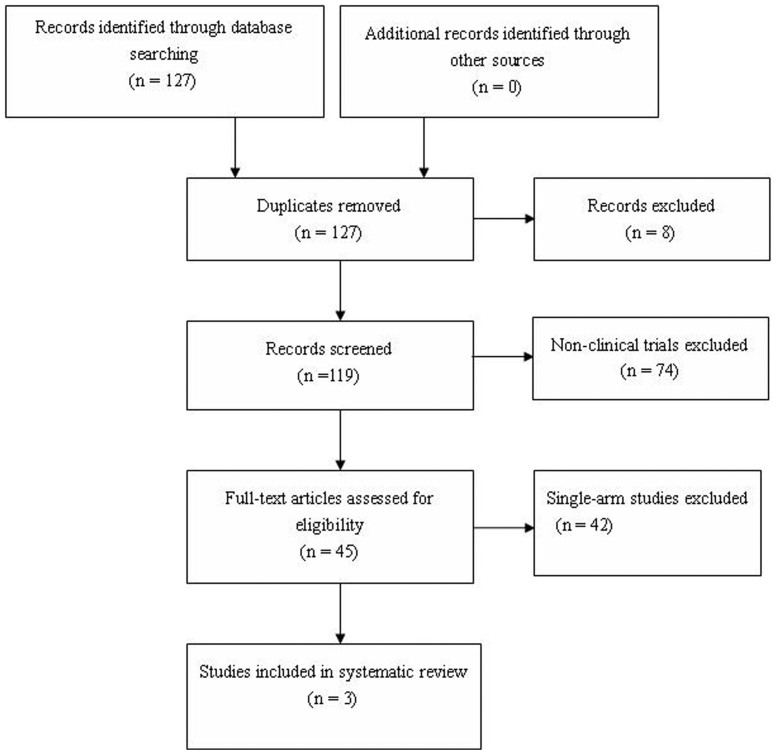
A total of 127 articles were identified through searching databases (Figure 1).After removing 8 duplicates and 74 non-clinical trials, the remaining 45 studies underwent full-text review, then 42 single-arm studies were excluded. Finally, three projects met the pre-defined criteria were enrolled in this systematic review6,7,17.
Figure 1. Flow chart of study selection.
Study characteristics
The characteristics of the studies included in the systematic review were summarized in Table 1 and patients' clinical data in Table 2. All three studies were randomly designed. In the study of Dohut et al6, patients received either docetaxel 30 mg/m2 intravenously every week for 3 consecutive weeks, followed by a 1-week rest period (n = 25); or docetaxel at the same dose and schedule, plus thalidomide 200 mg orally each day (n = 50). In the study of Sissung et al17, 73 men with AIPC treated with either single-agent docetaxel, administered intravenously at a dose of 30 mg/m2 (n = 23), or with docetaxel on the same schedule plus oral thalidomide at a dose of 200 mg orally each day (n = 50). In the study of Figg et al7, patients were randomized to receive either (1) docetaxel, 30 mg/m2 every week for 3 consecutive weeks, followed by a l-week rest period (ie, 4-week cycle) (n = 17); or (2) docetaxel, 30 mg/m2 every week for 3 consecutive weeks, plus thalidomide, 200 mg orally each day at bedtime (the first dose was administered for 1 week before the first docetaxel infusion), followed by a l-week rest period (ie, 4-week cycle) (n = 36).
Table 1. Characteristics of studies included in the review.
| Study | Study design | Patient population | Intervention | Comparison | Outcome |
|---|---|---|---|---|---|
| Sissung TM 2008 | phase II trial | Androgen-independent prostate cancer | docetaxel plus thalidomide | docetaxel | 1. Response to Therapy OS2. Toxicity |
| Dahut WL 2004 | phase II trial | Androgen-independent prostate cancer | docetaxel plus thalidomide | docetaxel | 1. Response to Therapy PSA decline PFS OS2. Toxicity3. Pharmacokinetics4. Changes of Circulating Angiogenic Growth Factor |
| Figg WD 2001 | phase II trial | Androgen-independent prostate cancer | docetaxel plus thalidomide | docetaxel | 1. Response to Therapy PSA decline2. Toxicity |
Table 2. Summary of therapeutic efficacy for docetaxel combined thlidomide vs. docetaxel alone in AIPC.
| Study | Regimen | Age(years) | Median OS (months) | Median PFS (months) | ≥50%PSA decline (Number) | Hematologic toxicities | Non-hematologic toxicities | ||
|---|---|---|---|---|---|---|---|---|---|
| Grade1–2 | Grade 3–5 | Grade1–2 | Grade 3–5 | ||||||
| Sissung TM 2008 | docetaxel plus thalidomide | N/A | N/A | N/A | N/A | N/A | 4(5%) | N/A | 1(1%) |
| docetaxel | N/A | N/A | N/A | ||||||
| Dahut WL 2004 | docetaxel plus thalidomide | 71(52–83) | 28.9 | 5.9 | 25/47 | 4(8%) | 7(14%) | 22(88%) | 9(18%) |
| docetaxel | 67(43–82) | 14.7 | 3.7 | 9/24 | 4(16%) | 7(28%) | 41(84%) | 2(8%) | |
| Figg WD 2001 | docetaxel plus thalidomide | 69(49–80) | N/A | N/A | 19/36 | N/A | 5(7%) | N/A | 1(1%) |
| docetaxel | N/A | N/A | 6/17 | ||||||
Abbreviation: OS: Overall survival; PFS: Progression-free survival; N/A: not applicable.
Survival
The prognosis is summarized in Table 2. Generally speaking, prognosis was more favorable in patients treated with docetaxel plus thalidomide than those treated with docetaxel alone.
Dahut et al6 and Figg et al7 reported response to therapy based on PSA, an over 50% decline in PSA occurred more frequently in the patients treated with docetaxel plus thalidomide than those treated with docetaxel alone. Dahut et al6 reported progression-free survival (PFS) and 18 month-OS rate, the results showed the median PFS in the combined group (5.7 months) was higher than that in docetaxel alone group (3.7 months, P = 0.32), and 18-month survival was 68.2% in the combined group, higher than that in docetaxel alone group (42.9%, P = 0.11).
Side-effects
All patients in three studies6,7,17 were evaluated for toxic effects. Dahut et al6 compared the frequency of the hematologic toxicities and non-hematologic toxicities in patients receiving different regimens, and the results showed that the hematologic toxicities were mild in both groups. As for the non-hematologic toxicitie, the incidence of depression, peripheral neuropathy and cardiac arrhythmia were slightly higher in the combined group than the docetaxel alone group. Sissung et al17 and Figg et al7 evaluated the toxic effects of all patients as a group, the results showed that they were more likely to experience hematologic toxicities and peripheral neuropathy than toxicities of other systems.
Discussion
In this systematic review, we analyzed the efficacy and safety of docetaxel plus thalidomide vs. docetaxel alone for handling AIPC. A total of three randomized studies, involving 201 patients, were included in the review. In general, the findings from these studies demonstrated superior survival in patients treated with docetaxel plus thalidomide, and all patients were more likely to experience hematologic toxicities and peripheral neuropathy than toxicities of other systems.
In the study of Dohut et al6, docetaxel plus thalidomide resulted in greater PSA decline, longer median PFS, and higher 18-month survival rate than docetaxel alone. Figg et al7 confirmed the better efficacy treated with docetaxel plus thalidomide in PSA decline than docetaxel alone. That might be due to the antiangiogenetic effect of thalidomide. Prostate tumors require angiogenic phenotype for progression to aggressive form15,16. Thalidomide inhibits angiogenesis, induces apoptosis in culture systems, and reduces higher levels of angiogenesis factors, such as VEGF and bFGF, that are present in patients with prostate cancer17. Thus, the antiangiogenetic effect of thalidomide combined regimen could possibly open up a promising new avenue for treating AIPC.
In terms of the toxicities of both regimens, the hematologic toxicities were mild while the non-hematologic toxicities, such as the incidence of depression, peripheral neuropathy and cardiac arrhythmia were slightly higher in the combined arm than the docetaxel alone arm. The results showed the combined regimen was well tolerated in the vast majority of patients. The addition of thalidomide might cause slightly higher non-hematologic toxicities, especially neuropathies, but the mechanism is still unknown.
We conducted a systematic review of the current literature to compare the efficacy and safety of docetaxel plus thalidomide vs. docetaxel alone for handling AIPC. Noticeably, the available evidence indicates potential survival advantage in docetaxel plus thalidomide over docetaxel alone. Futher well-designed and prospective studies are advisable.
Methods
Search strategy
PubMed, Biomedical Central, Google Scholar and Cochrane databases were searched using combinations of the following terms: prostate cancer/prostate carcinoma, docetaxel/taxotere, thalidomide. PubMed - prostate cancer AND docetaxel AND thalidomide (limits English), prostate carcinoma AND docetaxel AND thalidomide (limits English), prostate cancer AND taxotere AND thalidomide (limits English); Biomedical Central -prostate cancer AND docetaxel AND thalidomide; Google Scholar -prostate cancer AND docetaxel AND thalidomide; Cochrane -prostate cancer AND docetaxel AND thalidomide. The search was performed in Feb 10, 2014. Reference lists of retrieved studies were hand searched where appropriate to identify other potentially relevant studies.
Selection
Studies were included in the systemic review meeting the following inclusion criteria: they were randomized clinical trials and involved patients with AIPC. Studies were excluded for case reports, focusing on other type of prostate cancer, or lack of efficacies and toxities analyzing.
Data extraction
Data were extracted from eligible studies by two independent reviewers (L.C. and X.X.Q.). Any disagreement was resolved through consultation with the third reviewer (R.X.W.). The following data were extracted from studies that met the eligibility criteria: author details, year of publication, study design, therapeutic regimens, number of patients, age of patients, median survival, survival rate, median PFS, PFS rate, and PSA decline, and toxicity response.
Outcome measurement
The primary outcome was median survival in months. Secondary prognosis included survival rate, median PFS, PFS rate and PSA decline. Toxicity data are also summarized.
Acknowledgments
We thank Professor Jun Tian (Public Health School, Fujian Medical University) for assistance in data processing and analyzing.
Footnotes
The authors declare no competing financial interests.
Author Contributions All authors have contributed to this article. L.C., X.X.Q. and R.X.W. searched the databases, extracted the data, screened trials, and reformed the tables. L.C. and X.X.Q. appraised the quality of included trials and drafted the full text. X.H.X. were responsible for editing, and acted as an arbitrator and conceived the article. All authors reviewed the manuscript.
References
- Siegel R., Naishadham D. & Jemal A. Cancer statistics, 2013. CA Cancer J Clin 63, 11–30, 10.3322/caac.21166 (2013). [DOI] [PubMed] [Google Scholar]
- Petrylak D. Therapeutic options in androgen-independent prostate cancer: building on docetaxel. BJU Int 96 Suppl 2, 41–46, 10.1111/j.1464-410X.2005.05946.x (2005). [DOI] [PubMed] [Google Scholar]
- Sharifi N., Gulley J. L. & Dahut W. L. Androgen deprivation therapy for prostate cancer. JAMA 294, 238–244, 10.1001/jama.294.2.238 (2005). [DOI] [PubMed] [Google Scholar]
- Oh W. K., Tully P., Kantoff P. W. & Regan M. M. Physician attitudes toward cytotoxic chemotherapy use in patients with advanced prostate carcinoma. Cancer 97, 2171–2179, 10.1002/cncr.11344 (2003). [DOI] [PubMed] [Google Scholar]
- Oh W. K. Chemotherapy for patients with advanced prostate carcinoma: a new option for therapy. Cancer 88, 3015–3021 (2000). [DOI] [PubMed] [Google Scholar]
- Dahut W. L. et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol 22, 2532–2539, 10.1200/JCO.2004.05.074 (2004). [DOI] [PubMed] [Google Scholar]
- Figg W. D. et al. A randomized phase II trial of docetaxel (taxotere) plus thalidomide in androgen-independent prostate cancer. Semin Oncol 28, 62–66 (2001). [DOI] [PubMed] [Google Scholar]
- Logothetis C. J. Docetaxel in the integrated management of prostate cancer. Current applications and future promise. Oncology (Williston Park) 16, 63–72 (2002). [PubMed] [Google Scholar]
- Berry W., Dakhil S., Gregurich M. A. & Asmar L. Phase II trial of single-agent weekly docetaxel in hormone-refractory, symptomatic, metastatic carcinoma of the prostate. Semin Oncol 28, 8–15 (2001). [DOI] [PubMed] [Google Scholar]
- Petrylak D. P. Docetaxel (Taxotere) in hormone-refractory prostate cancer. Semin Oncol 27, 24–29 (2000). [PubMed] [Google Scholar]
- D'Amico A. V., Chen M. H., Roehl K. A. & Catalona W. J. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med 351, 125–135, 10.1056/NEJMoa032975 (2004). [DOI] [PubMed] [Google Scholar]
- Ward J. F., Blute M. L., Slezak J., Bergstralh E. J. & Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol 170, 1872–1876, 10.1097/01.ju.0000091876.13656.2e (2003). [DOI] [PubMed] [Google Scholar]
- Stephenson A. J. et al. Utility of PSA doubling time in follow-up of untreated patients with localized prostate cancer. Urology 59, 652–656 (2002). [DOI] [PubMed] [Google Scholar]
- Mellin G. W. & Katzenstein M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N Engl J Med 267, 1184–1192, contd, 10.1056/NEJM196212062672305 (1962). [DOI] [PubMed] [Google Scholar]
- Singhal S. et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 341, 1565–1571, 10.1056/NEJM199911183412102 (1999). [DOI] [PubMed] [Google Scholar]
- Siegal J. A., Yu E. & Brawer M. K. Topography of neovascularity in human prostate carcinoma. Cancer 75, 2545–2551 (1995). [DOI] [PubMed] [Google Scholar]
- Sissung T. M. et al. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res 14, 4543–4549, 10.1158/1078-0432. (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]